Session Information
Date: Sunday, November 8, 2020
Title: Pediatric Rheumatology – Clinical I: Treatment of JIA (1492–1496)
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose: Tofacitinib is an oral JAK inhibitor that is being investigated for JIA. We report the safety, tolerability, and efficacy of tofacitinib in patients (pts) with JIA in an open-label, long-term extension study with up to 66 months of observation.
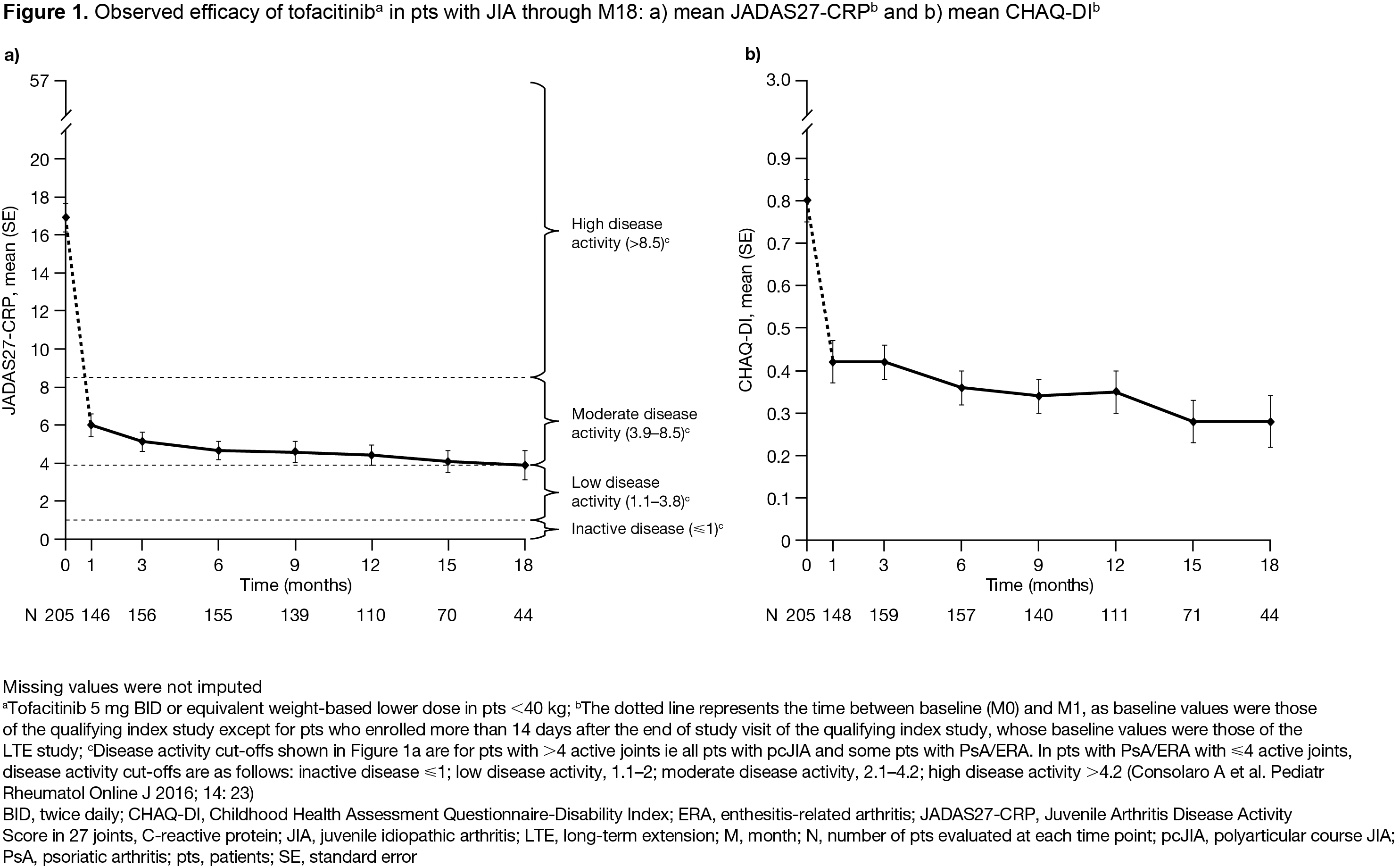
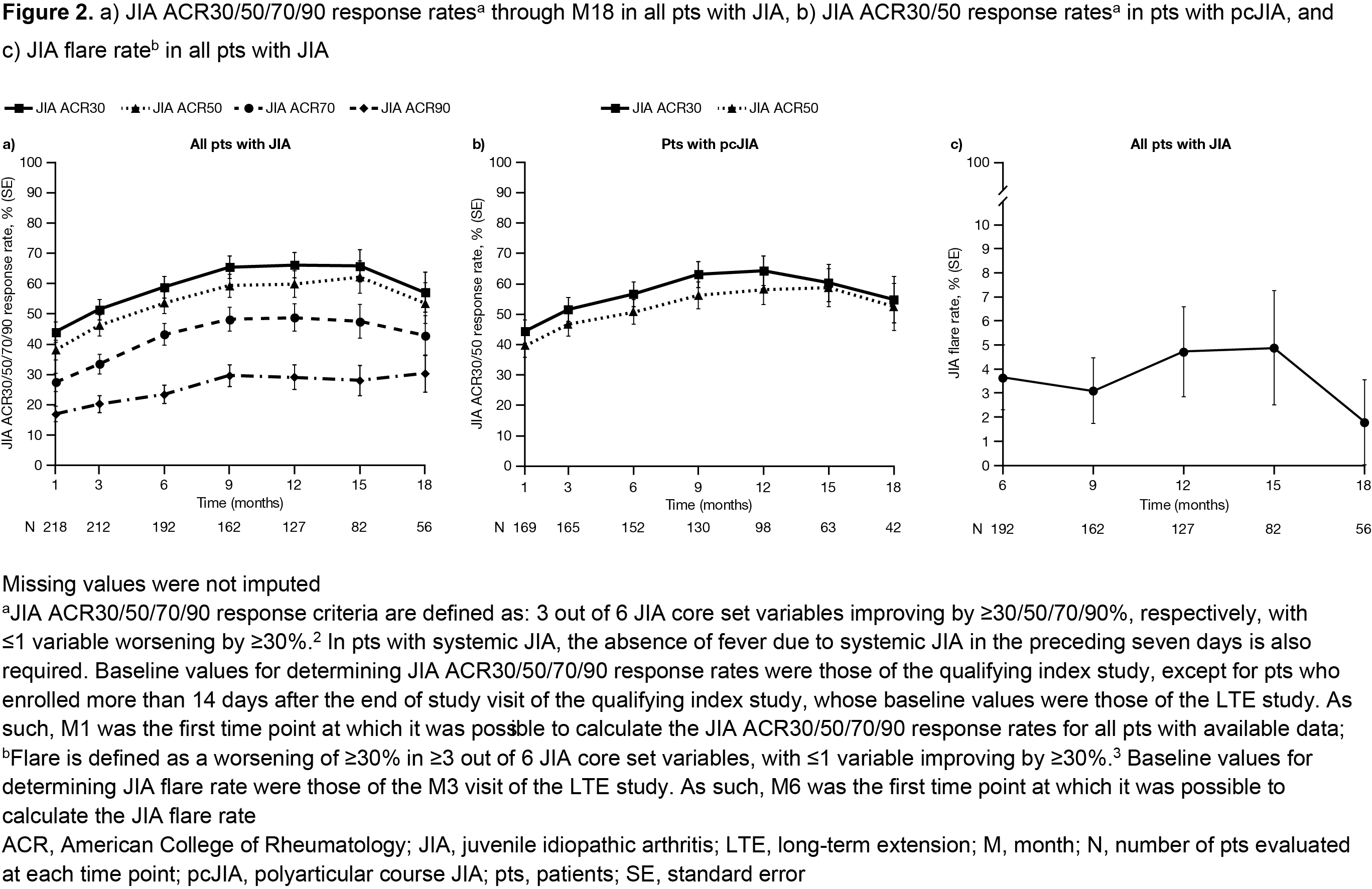
Methods: We performed an interim analysis of this ongoing study (NCT01500551; data cut-off: June 4, 2019; database not locked and subject to change). Eligible pts aged 2–≤ 18 years with polyarticular course (pc)JIA, juvenile (j)PsA, or ERA were enrolled after completing a Phase (P)1 (NCT01513902) or P3 (NCT02592434) tofacitinib index study, or after discontinuing from the index studies for reasons other than treatment-related serious adverse events (SAEs). Pts received open-label tofacitinib 5 mg BID or an equivalent weight-based lower dose. Safety endpoints, reported up to Month (M)66 for the overall cohort, were AEs (including AEs of special interest and laboratory test abnormalities) and active uveitis. Efficacy endpoints, reported through M18 for the overall cohort, included mean JADAS27-CRP, JADAS27-CRP minimal disease activity (scores ≤ 3.8 and ≤ 2 in pts with > 4 and ≤ 4 active joints, respectively1) rate, mean CHAQ‑DI, JIA ACR30/50/70/90 response2 rates, JIA ACR30/50 response rates for pts with pcJIA, JIA ACR clinical remission (ie inactive disease for 6 months continuously), and JIA flare3 rate.
Results: 223 pts with pcJIA (n=172), jPsA (n=19), or ERA (n=21) were enrolled and received open-label tofacitinib (total follow-up 265.8 pt-years). Most pts were female (74.9%) and mean (range) age was 12.3 (3−18) years. Mean (median; range) tofacitinib exposure was 402.4 (347.0; 20.0−1,983.0) days. Over 66 months, 160 (71.7%) pts had AEs; 15 (6.7%) pts had SAEs; 13 (5.8%) pts discontinued due to AEs (Table). The most common AEs by MedDRA preferred term were upper respiratory tract infection (16.1% of pts), JIA exacerbation (8.5% of pts), and nasopharyngitis (8.5% of pts). Five (2.2%) pts had serious infections. Two (0.9%) pts had herpes zoster: one case was non-serious; the other case was serious and adjudicated as opportunistic. Laboratory test abnormality rates were ≤ 2.7% (Table). One (0.4%) pt at M12 had active uveitis. Improvements in disease activity (JADAS27-CRP; Figure 1a) and physical functioning (CHAQ-DI; Figure 1b) were maintained through M18. In M1−18, JADAS27-CRP minimal disease activity rates were > 50%. JIA ACR30/50 response rates overall, and in pts with pcJIA, increased from M1 and were > 50% from M6−18 (Figure 2a/b). JIA ACR70/90 response rates overall increased from M1 and were > 40% and > 20%, respectively, from M6−18 (Figure 2a). None of the 223 pts achieved JIA ACR clinical remission by M18. JIA flare rate was < 5% through M18 (Figure 2c).
Conclusion: In this open-label, long-term extension study of tofacitinib in pts with JIA, no new safety findings were observed over 66 months. Clinical efficacy was maintained over 18 months.
- Consolaro A et al. Arthritis Rheum 2012; 64: 2366-2374.
- Giannini EH et al. Arthritis Rheum 1997; 40: 1202-1209.
- Brunner HI et al. J Rheumatol 2002; 29: 1058-1064.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Gemma Turner, CMC Connect, and funded by Pfizer Inc.
To cite this abstract in AMA style:
Brunner H, Akikusa J, Al-Abadi E, Bohnsack J, Boteanu A, Chedeville G, Cuttica R, De La Pena W, Jung L, Kasapcopur O, Kobusinska K, Schulert G, Neiva C, Rivas-Chacon R, Cruz Rizo Rodriguez J, Vazquez-Del Mercado M, Wagner-Weiner L, Weiss J, Wouters C, Suehiro R, Posner H, Wouters A, Kanik K, Luo Z, Martini A, Lovell D, Ruperto N. Tofacitinib for the Treatment of Patients with Juvenile Idiopathic Arthritis: An Interim Analysis of Data up to 5.5 Years from an Open-label, Long-term Extension Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/tofacitinib-for-the-treatment-of-patients-with-juvenile-idiopathic-arthritis-an-interim-analysis-of-data-up-to-5-5-years-from-an-open-label-long-term-extension-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tofacitinib-for-the-treatment-of-patients-with-juvenile-idiopathic-arthritis-an-interim-analysis-of-data-up-to-5-5-years-from-an-open-label-long-term-extension-study/