Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 11:30AM-1:00PM
Background/Purpose: The efficacy of interleukin-6 receptor blockade in hospitalized COVID-19 patients not on mechanical ventilation is unclear.
Methods: We performed a randomized, double-blind, placebo-controlled trial in patients with patients confirmed SARS-CoV-2 infection, elevated serum inflammatory markers, and at least two of: 1) pulmonary infiltrate; 2) supplemental oxygen requirement to maintain oxygen saturation >92%; or, 3) fever >38°C. Patients were randomized 2:1 to standard care plus intravenous tocilizumab 8mg/kg or placebo. The primary outcome was time to mechanical ventilation or death during the 28-day study period. The two secondary efficacy outcomes were time to clinical worsening and time to resolution of supplemental oxygen requirement.
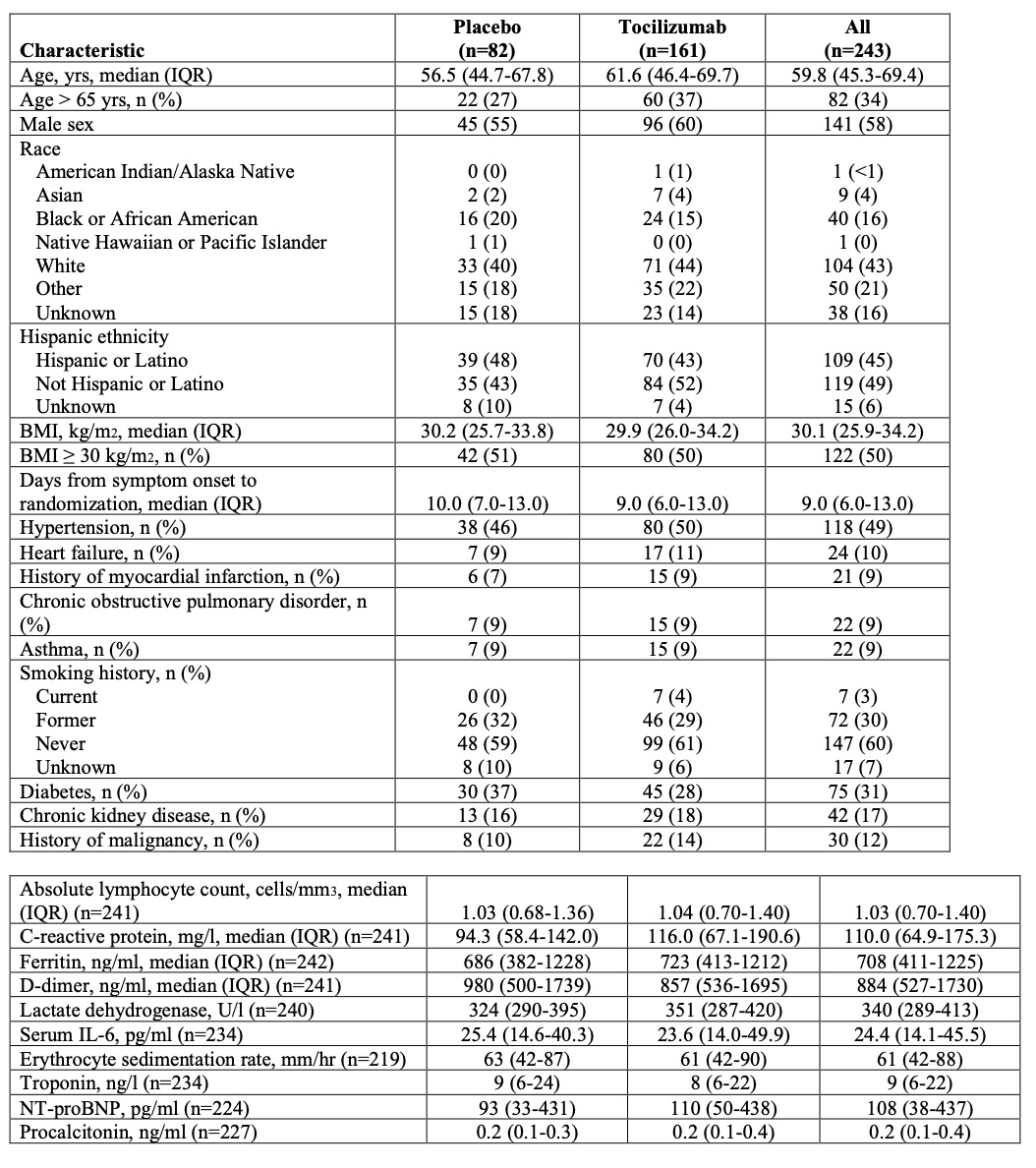
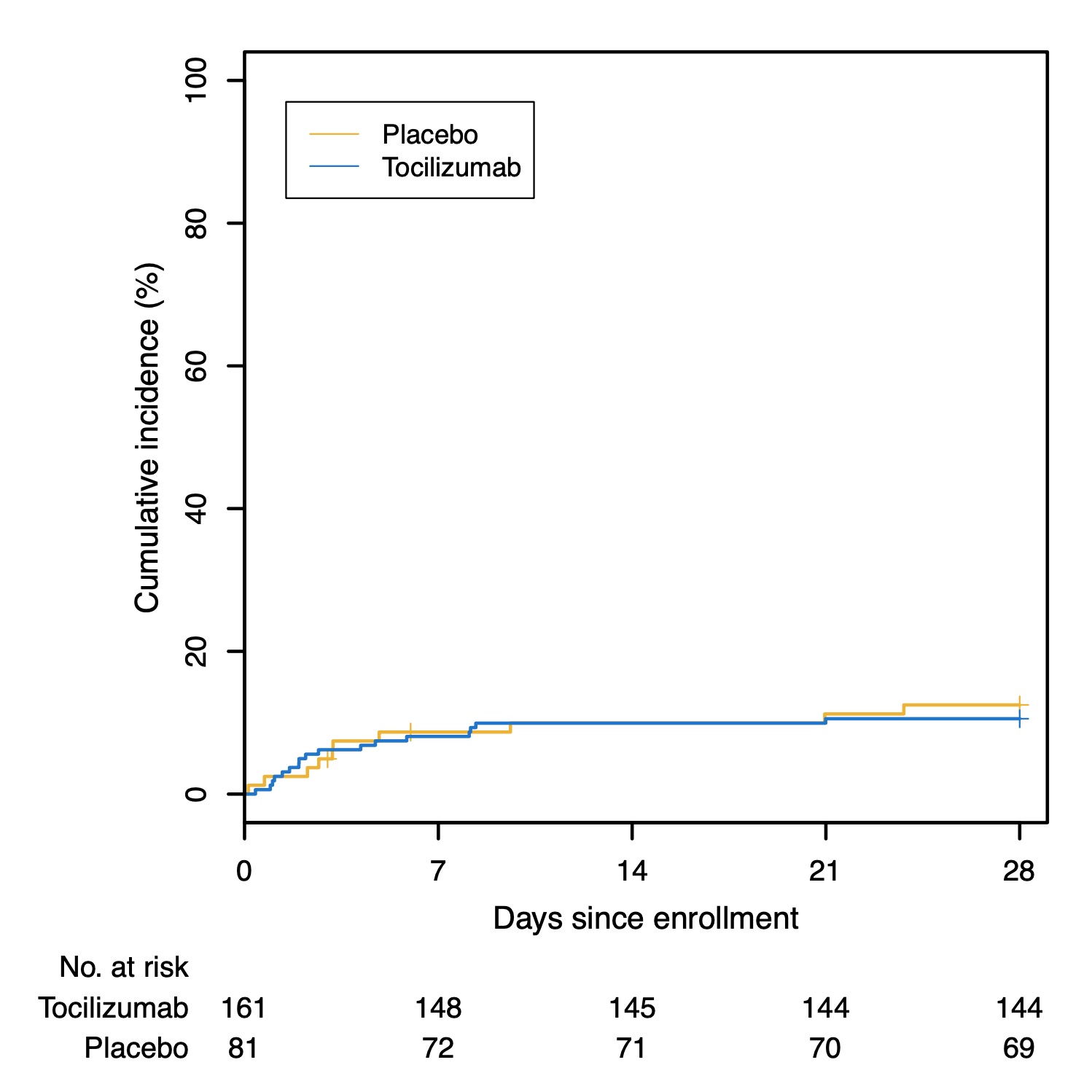
Results: Two-hundred forty-three patients were enrolled. One-hundred forty-one (58%) were men, 102 (42%) women. Median age was 58.8 years (range 21.7-85.4 years). Forty-five percent self-identified as Hispanic/Latino. The hazard ratio (HR) for progression to intubation or death in the tocilizumab group was 0.83 (95% confidence interval [CI] 0.38, 1.81; P=0.64). The HR for disease worsening was 1.11 (95% CI 0.59, 2.10; P=0.73) in the tocilizumab group. At 14 days, 18.0% versus 14.9% (tocilizumab versus placebo) had experienced disease worsening. The median time to oxygen discontinuation was 5.0 days (3.8, 7.6 days) in the tocilizumab group and 4.9 days (3.8, 7.8 days) with placebo (P=0.69). At 14 days, 24.6% of tocilizumab-treated patients still required oxygen, compared with 21.2% in the placebo group. Tocilizumab-treated patients had fewer serious infections.
Conclusion: Interleukin-6 receptor blockade is not effective for preventing mechanical ventilation or death among moderately ill patients with COVID-19 infection.
To cite this abstract in AMA style:
Stone J. Tocilizumab for COVID-19 Infection: A Randomized, Double-Blind, Placebo-Controlled Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/tocilizumab-for-covid-19-infection-a-randomized-double-blind-placebo-controlled-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-for-covid-19-infection-a-randomized-double-blind-placebo-controlled-trial/