Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Tocilizumab (TCZ) is used in diseases characterized by markedly elevated inflammatory markers and elevated pro-inflammatory cytokines like rheumatoid arthritis (RA), juvenile idiopathic arthritis and giant cell arteritis. Complement system has been implicated in rheumatoid arthritis (RA) etiopathogenesis. The aim of this work was to analyze how TCZ influences the three pathways of complement system.
Methods: Twenty-seven RA patients included in the TOCRIVAR study who received TCZ (8mg/kg IV/q4w) were evaluated at baseline and at weeks 12, 24 and 52 of treatment. Disease activity, acute phase reactants and new generation functional assays of the three routes of complement system were assessed at baseline and at each visit. Multivariable linear mixed models were performed to study changes over time in the complement system cascades.
Results: A total of 27 RA patients, consisting of 24 females and 3 males with a mean age of 52 ± 11 years, were included in this study. The median disease duration was 8 years (IQR 2–12), and 69% tested positive for both anti-citrullinated protein antibodies (ACPA) and rheumatoid factor. The patients exhibited active disease as indicated by DAS28-ESR (5.77 ± 0.88), SDAI (29 ± 10), and CDAI (27 ± 10) scores. Twenty patients (77%) were on prednisone, and 19 (70%) were receiving tocilizumab (TCZ) in combination with either methotrexate or leflunomide. Only 8 patients (30%) were on TCZ monotherapy.
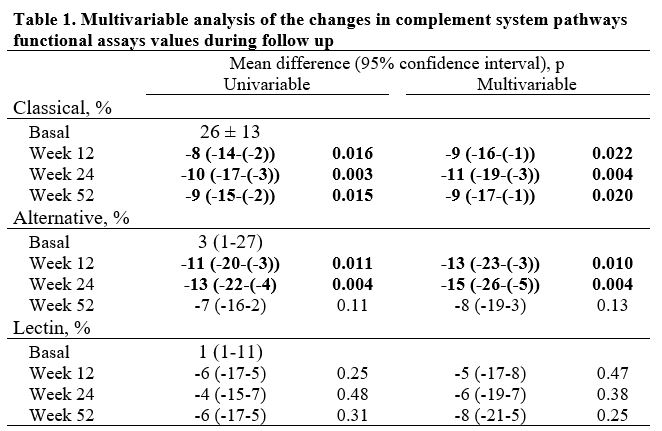
BMI and waist circumference remained stable and did not reveal any differences after one year of treatment compared to baseline values. As expected, during follow-up acute-phase reactants and disease activity scores improved over time. The progression of functional complement tests following TCZ treatment over a year is shown in Table 1. The classical pathway exhibited significant decreases at weeks 12, 24, and 52 compared to baseline. These changes remained significant after multivariable adjustment using the CDAI index. The alternative pathway showed significant decreases at weeks 12 and 24, but not at 52 weeks; these changes also remained significant after adjusting for disease activity. Conversely, the functional test for the lectin pathway showed no changes throughout the follow-up period (Table 1).
Conclusion: TCZ decreases values of the functional assays of the classical and alternative routes of the complement system. This effect is independent of the reduction that TCZ exert over disease activity.
Multivariable analysis is adjusted for CDAI (Clinical Disease Activity Index).
Differences are shown using basal value of each route assay as the reference category.
To cite this abstract in AMA style:
Hernández-Díaz M, Ferraz-Amaro I, Santos-Concepción S, Castro J, Hernandez V, Tejera Segura B, Luna C, Delgado-Frías E, Diaz-González F. Tocilizumab Effect on the Three Pathways of the Complement System in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/tocilizumab-effect-on-the-three-pathways-of-the-complement-system-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-effect-on-the-three-pathways-of-the-complement-system-in-patients-with-rheumatoid-arthritis/