Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: SLE is a chronic disease in which irreversible organ damage can accumulate over time; treatments associated with rapid responses in patients are therefore desirable.1 Pooled data from the phase 3 TULIP trials support that the approved monoclonal antibody anifrolumab reduces moderate to severe SLE activity across multiple organ systems when added to standard therapy over 52 weeks.2 Here, we investigate the kinetics of disease activity improvements over 52 weeks for individual SLEDAI-2K items and organ systems in the TULIP trials.
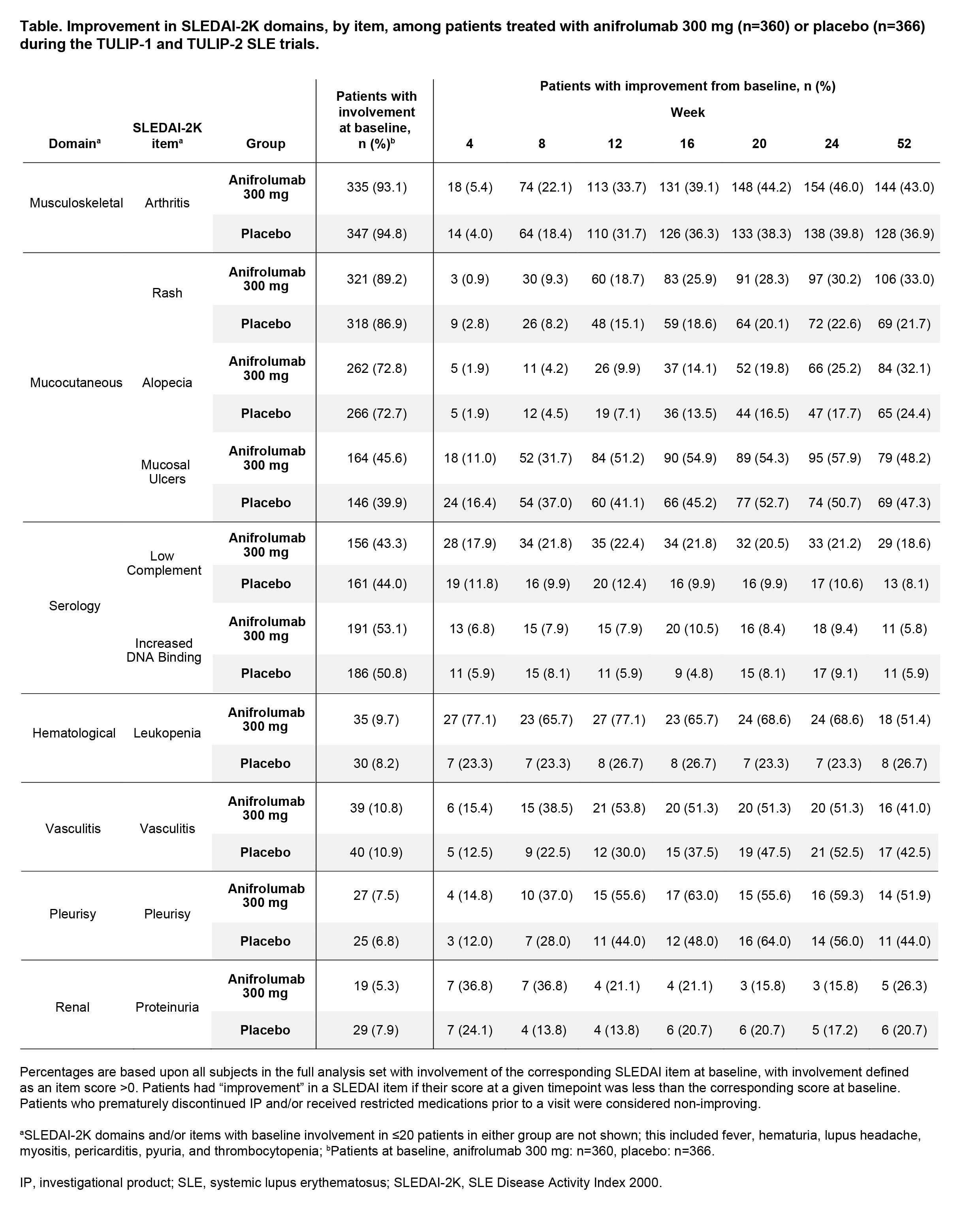
Methods: We included adults with moderate to severe SLE receiving intravenous anifrolumab 300 mg or placebo every 4 weeks (Q4W) alongside standard therapy in the randomized, double-blind, placebo-controlled phase 3 trials TULIP-1 and -2 (NCT02446912, NCT02446899).2 Patient status for each SLEDAI-2K item was assessed at each visit; items reported here had baseline involvement in ≥20 patients in either treatment group. The proportions of patients with SLEDAI-2K individual item improvements were calculated by treatment group and visit; improvement was defined as a reduction vs baseline score. Numeric differences between treatment groups ≥10% are described.
Results: Individual SLEDAI-2K items were assessed in 726 patients (anifrolumab: n=360; placebo: n=366); baseline involvement was generally balanced between treatment groups (Table). From Week 4 onwards, numerically greater proportions of patients had leukopenia improvement with anifrolumab vs placebo (Week 4: 77.1% vs 23.3%). From Week 8 onwards, a numeric difference favoring anifrolumab vs placebo was seen in the proportion of patients with complement improvement (Week 8: 21.8% vs 9.9%). Though the proportions of patients with improvement were similar from Weeks 20–52, numeric treatment differences favoring anifrolumab vs placebo were seen at Weeks 8–16 for vasculitis (Week 8: 38.5% vs 22.5%), and Weeks 12–16 for mucosal ulcers (Week 12: 51.2% vs 41.1%) and pleurisy (Week 12: 55.6% vs 44.0%, note small subgroup sizes). Increased anti-dsDNA binding improved in ≤20 patients per treatment group at any timepoint, with no consistent differences over time. The proportions of patients with improvements in mucocutaneous domain items generally increased over time in both treatment groups; more than 25% of patients improved with anifrolumab, with small numeric differences (< 10%) favoring anifrolumab vs placebo from Week 16 for rash and Week 24 for alopecia.
Conclusion: A desired feature of SLE treatments is for patients to rapidly experience measurable improvements in disease activity. More patients saw early improvements in mucosal ulcers, vasculitis, and pleurisy, with anifrolumab vs placebo, despite similar treatment differences by Week 52. Normalization of leukopenia and low complement SLEDAI-2K items—two markers of disease activity routinely measured in clinical practice—were observed after just two doses of Q4W anifrolumab vs placebo.
1. Fanouriakis A. Ann Rheum Dis. 2024;83(1):15–29.
2. Morand EF. Lancet Rheumatol. 2022;4:e282–92.
To cite this abstract in AMA style:
Vital E, Furie R, Morand E, Bruce I, Knagenhjelm J, Lindholm C. Timing of SLEDAI-2K Item Improvements During the First Year of Intravenous Anifrolumab Treatment of Moderate to Severe SLE [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/timing-of-sledai-2k-item-improvements-during-the-first-year-of-intravenous-anifrolumab-treatment-of-moderate-to-severe-sle/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/timing-of-sledai-2k-item-improvements-during-the-first-year-of-intravenous-anifrolumab-treatment-of-moderate-to-severe-sle/