Session Information
Date: Tuesday, November 14, 2023
Title: Plenary III
Session Type: Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Three-quarters of RA patients use glucocorticoids (GC) to manage RA symptoms. Prior work suggests recent GC use is associated with major adverse cardiovascular events (MACE) in RA, but these studies did not adequately consider the effect of prior GC exposure on current risk.
Methods: In this retrospective cohort study, we used national VA administrative data to identify RA patients with an initial VA rheumatology visit in 2010-2018 (index date) and a one-year lookback period prior to this. Exclusions included age < 40 or >90, non-RA rheumatologic disorder, prior MACE, or CHF. We used a Cox proportional hazards model to estimate the effect of time-varying cumulative weighted oral GC use (primary exposure) on risk of MACE defined as MI, stroke/TIA, cardiac arrest, coronary revascularization, or death from CV cause (primary outcome). We estimated the primary exposure using a weighted cumulative dose (WCD) model representing GC exposure as a weighted sum of past doses (Sylvestre et al, 2009). Weights estimated using cubic regression splines reflect the relative impact of GC exposure at different times. Since the time period over which prior GC exposure affects MACE risk is not well defined, we fit potential WCD models evaluating exposure over the 30 days, 90 days, 180 days, 1 year, 2 years, and 3 years prior to MACE. The best-fitting model, using a 2-year exposure window prior to MACE, was selected (Fig 1).
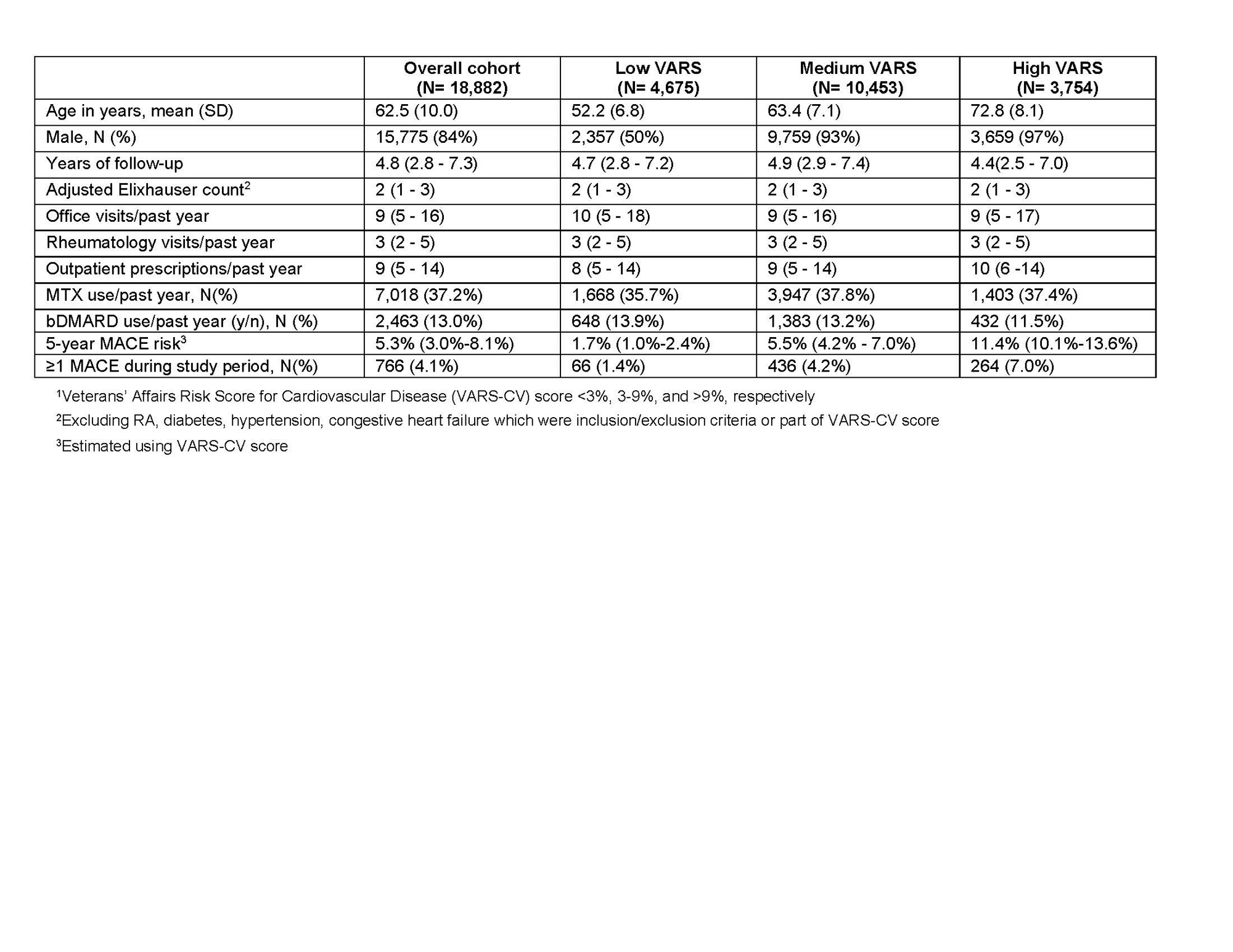
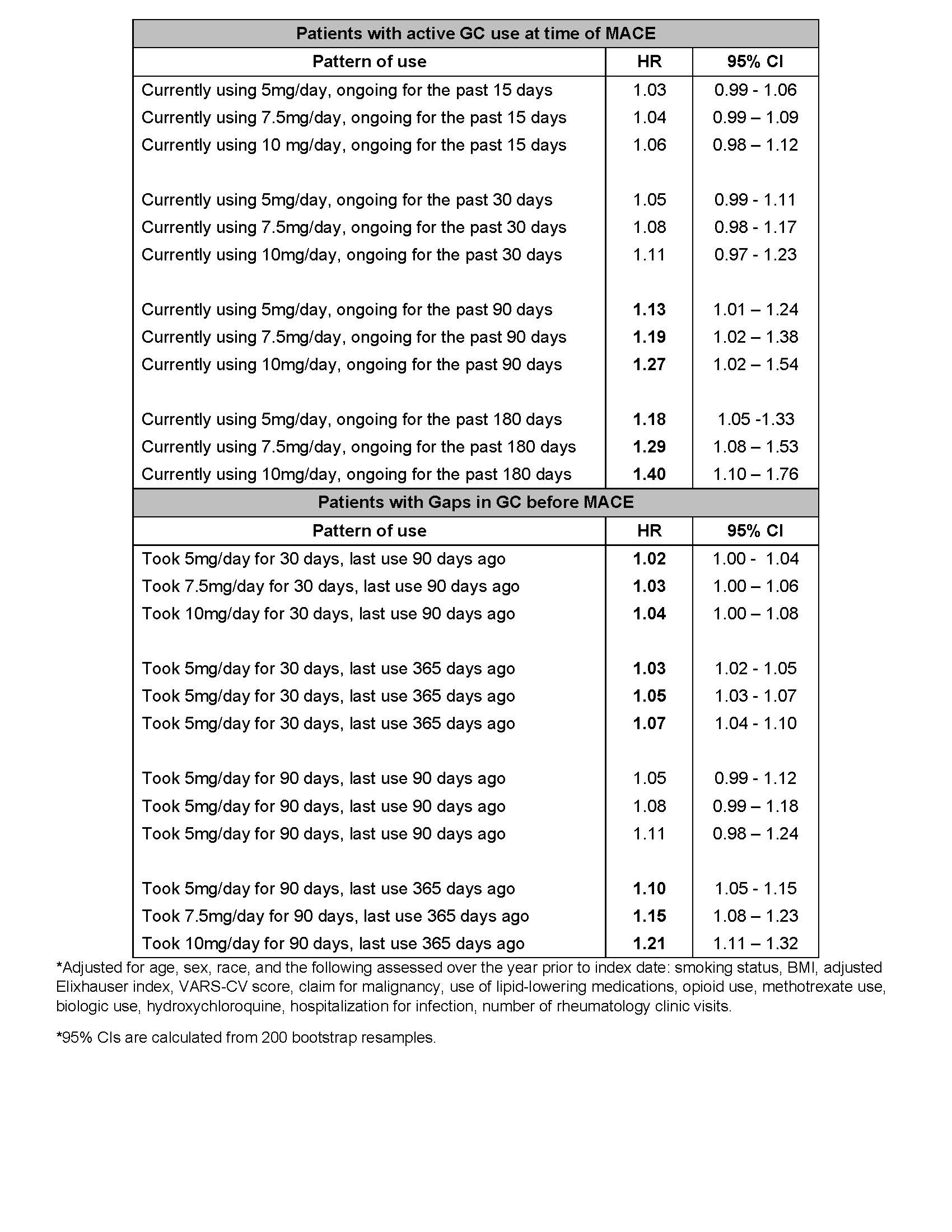
Results: Among 18,882 patients (mean age 62.5 years, 84% male), median 5-year MACE risk at baseline was 5.3%; 3,754 patients (19.9%) had high baseline MACE risk (Table 1). Incident MACE occurred in 4.1% of patients, with median time to MACE of 2.67 (IQR 1.26-4.45) years. Table 2 shows adjusted hazard ratios (aHRs) for several temporal patterns of GC exposure compared to no use for the 2 years prior to MACE. Using 5mg, 7.5mg, and 10mg/day prednisone equivalent for 90 days prior to MACE is associated with a 13%, 19%, and 27% increase in MACE, respectively (aHR [95% CI] 1.13 [1.01-1.24], 1.19 [1.02-1.38], 1.27 [1.02-1.54]). aHRs and 95% CI for use of 5mg/day for 15 and 30 days prior to MACE were 1.03 (0.99-1.06) and 1.05 (0.99-1.11) respectively. Use of 5mg, 7.5mg, and 10mg for 30 days, with last use 1 year pre-MACE, were associated with 3%, 5%, and 7% increases in MACE (aHR [95% CI] 1.03 [1.02-1.05], 1.05 [1.03-1.07], 1.07 [1.04-1.10]. Similar use for 90 days was associated with 10%, 15%, and 21% increases, respectively (aHR [95% CI] 1.10 [1.05-1.15], 1.15 [1.08-1.23], 1.21 [1.11-1.32]).
Conclusion: In this national RA cohort, we observed a dose-, duration-, and recency-dependent relationship between previous GC use and MACE. GC doses as small as 5mg/day, durations as short as 30 days, and use as long as one year prior to MACE were all associated with an increased risk of MACE. Future work will examine the effect of time-varying covariate adjustment, and potential effect modification of prior GC exposure on risk associated with current use.
To cite this abstract in AMA style:
Wallace B, Gao Y, Kim H, England B, Baker J, Sauer B, Cannon G, Roul P, Mikuls T, Cohen-Mekelburg S, Clauw D, Wiitala W, Hayward R, Sussman J, Waljee A. Time-Dependent Evaluation of Weighted Cumulative Glucocorticoid Exposure and Major Adverse Cardiovascular Events in a Cohort of Veterans with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/time-dependent-evaluation-of-weighted-cumulative-glucocorticoid-exposure-and-major-adverse-cardiovascular-events-in-a-cohort-of-veterans-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/time-dependent-evaluation-of-weighted-cumulative-glucocorticoid-exposure-and-major-adverse-cardiovascular-events-in-a-cohort-of-veterans-with-rheumatoid-arthritis/