Session Information
Date: Sunday, November 10, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster I: Risk Factors, Predictors, & Prognosis
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: ACPA positive individuals with musculoskeletal symptoms are at increased risk of developing RA. In such individuals, ACPA are conventionally identified on the basis of a positive serum IgG anti-CCP2 antibody (Ab) test.
Third-generation anti-CCP (CCP3) tests (Inova Diagnostic) have been recently introduced and their diagnostic value has been evaluated in patients with early and established RA.
The value of anti-CCP3 for predicting progression to RA in at-risk individuals is less understood. Consequently, the main aims of the study were:
- to determine the prevalence of anti-CCP3 Ab in anti-CCP2 positive at-risk individuals and the agreement between the 2 tests;
- to investigate the association between anti-CCP3 Ab and progression to RA;
- to explore the stability of anti-CCP3 Ab status over time.
Methods: Anti-CCP3 Ab were tested on stored serum samples obtained from 337 anti-CCP2 positive (BioRad, USA) at-risk individuals without synovitis from the Leeds CCP study.
Anti-CCP2 and anti-CCP3 tests positivity threshold was >2.99 IU/ml and >20 units, respectively. Anti-CCP2 and anti-CCP3 Ab were considered low titre (LT) or high titre (HT) if < or > than 3 times the positivity threshold, respectively.
Only subjects with at least one follow-up visit were included in the progression analysis (n=308). Sequential samples were tested for CCP3 in 132 individuals.
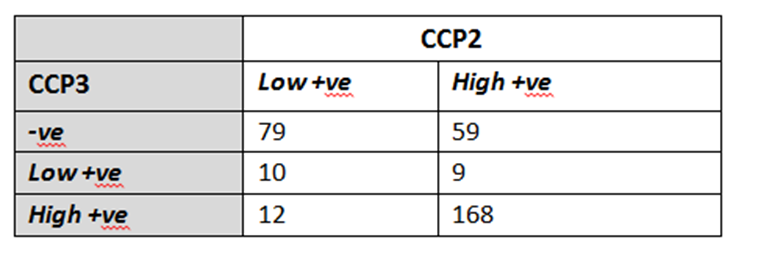
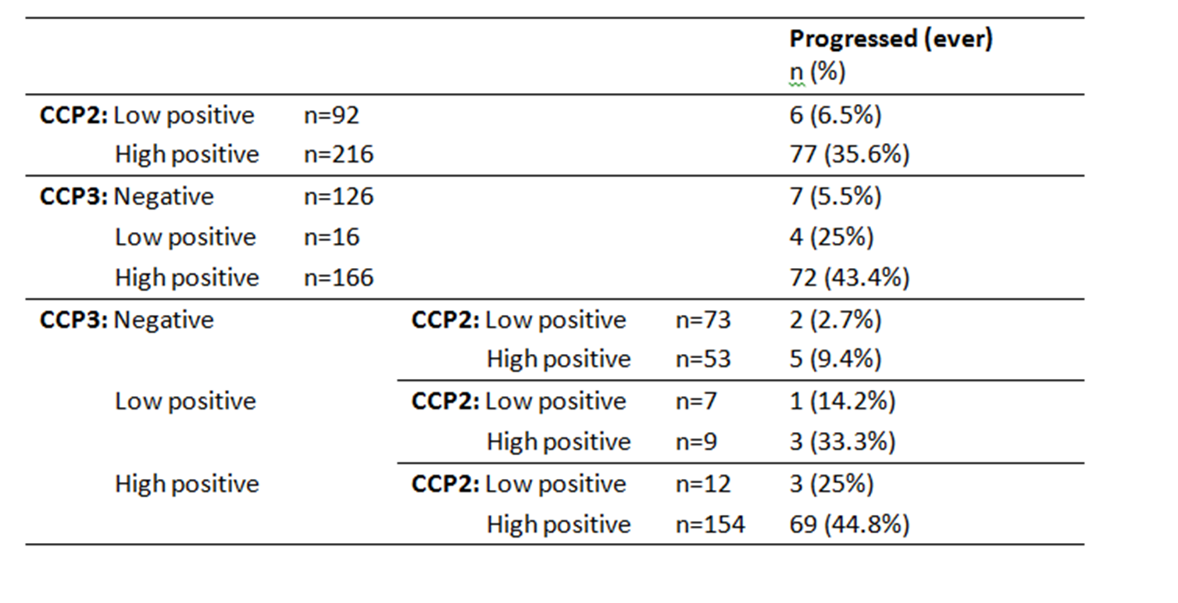
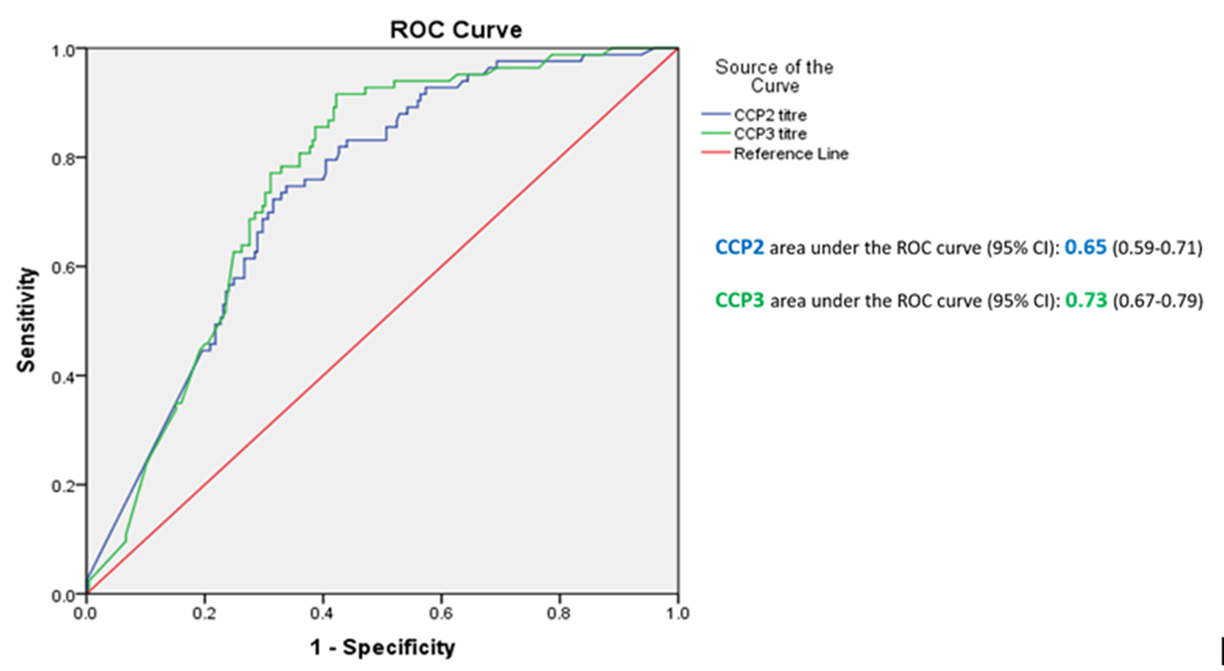
Results: Anti-CCP3 Ab tended to be either negative (138/337; 40.9%) or HT positive (180/337; 53.4%), with a few subjects showing a LT (19/337; 5.6%). In contrast, for anti-CCP2, more LT were observed (101/337; 30%). The Cohen’s k agreement between anti-CCP2 and anti-CPP3 test was 0.22 (0.17-0.26) (p< 0.001) (Table 1). Eighty-three/308 subjects (27%) developed arthritis (median follow up 273 days, min 3 – max 3402), 73 of whom fulfilled 2010 ACR/EULAR classification criteria for RA. The proportions of patients progressing to arthritis (ever) according to anti-CPP2 and anti-CPP3 Ab status are illustrated in Table 2. The rate of progression of LT and HT anti-CCP2, when anti-CCP3 was negative, fell from 6.5% to 2.7%, and from 45.6% to 9.4%, respectively. Progression in anti-CCP2 HT increased from 35.6% to 44.8%, when anti-CCP3 was positive. The hazard ratio for HT anti-CCP2 and HT anti-CPP3 Ab was 4.9 (CI 2.1-11.2) and 6.9 (CI 3.1-15.0) (p< 0.001), respectively. The ROC curves for anti-CCP2 and anti-CCP3 tests are shown in Figure 1.
At baseline, 33/132 (25%) individuals who had CCP3 tested at ≥1 timepoint for sequential samples were anti-CCP3 negative and 99/132 (75%) were anti-CCP3 positive (5 LT, 94 HT). The anti-CCP3 Ab titer remained stable in 125/132 (94.7%) individuals, in 559/575 (97.6%) sequential samples (mean follow-up 551 days ±623.53).
Conclusion: The distributions of anti-CCP2 and anti-CCP3 assays differed and their agreement was poor.
Our results suggest a potential value of anti-CCP3 antibodies in improving prediction of clinical arthritis in both LT and HT CCP2 positive at-risk subjects.
To cite this abstract in AMA style:
Di Matteo A, Mankia K, Duquenne L, Garcia-Montoya L, Corscadden D, Mbara K, Nam J, Mahler M, Emery P. Third Generation Anti-cyclic Citrullinated Peptide Antibodies Improve Prediction of Clinical Arthritis in Second Generation Anti-cyclic Citrullinated Peptide Positive Subjects at Risk of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/third-generation-anti-cyclic-citrullinated-peptide-antibodies-improve-prediction-of-clinical-arthritis-in-second-generation-anti-cyclic-citrullinated-peptide-positive-subjects-at-risk-of-rheumatoid-ar/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/third-generation-anti-cyclic-citrullinated-peptide-antibodies-improve-prediction-of-clinical-arthritis-in-second-generation-anti-cyclic-citrullinated-peptide-positive-subjects-at-risk-of-rheumatoid-ar/