Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Osteoarthritis (OA) is severe and intractable musculoskeletal disease that eventually leads to joint failure and pain due to inflammation and joint injury. Mesenchymal stem cells (MSCs) are expected to repair damaged tissues due to their multipotency for differentiation and immunomodulatory properties. MSCs have been the promising treatment source for osteoarthritis. However, few studies reported about outcomes of an intra-articular (IA) injection of allogenic bone marrow derived MSCs (BM-MSCs). This study aimed to assess the efficacy and safety of a single IA injection of BM-MSCs for patients with knee OA.
Methods: We performed a randomized, double-blind, placebo-controlled study. Twenty-four knee OA patients were randomized to receive single IA injection of MSCs (1×108 cells/ 2 mL) or normal saline (2 mL). The change of patient reported outcome measures (PROMs) including the visual analogue scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and Knee Injury and Osteoarthritis Outcome Score (KOOS) after IA injection were assessed at serial 3, 6, 9, and 12 months from baseline. Furthermore, we performed imaging studies using magnetic resonance (MR) with T2 mapping sequences to analyze knee cartilage in all patients at baseline, 3 months, and 12 months.
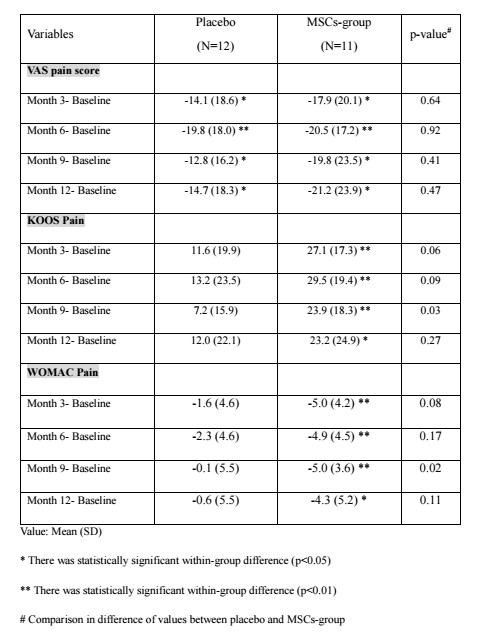
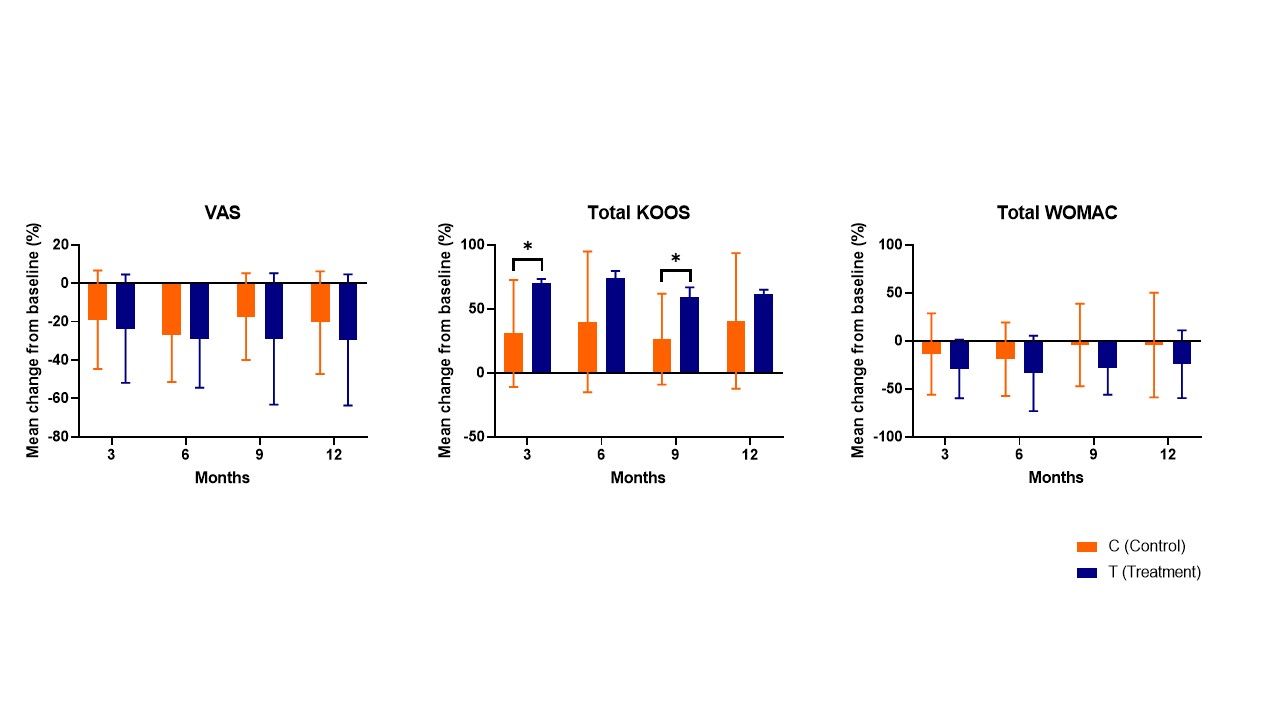
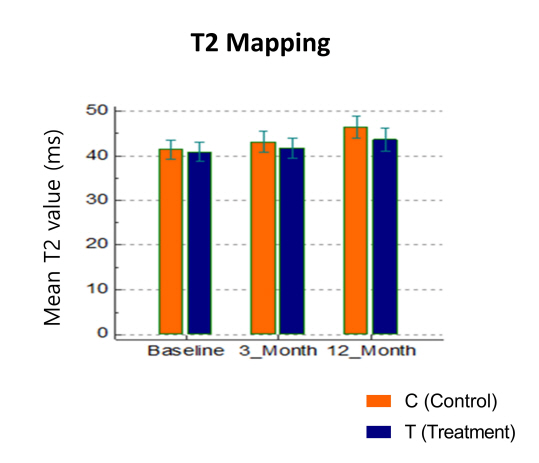
Results: A total of twenty-three patients entered the final analysis, with eleven in MSCs treatment group and twelve in control group. Within group improvement of pain, measured by KOOS and WOMAC, when compared with baseline was statistically significant throughout all time points only in treatment group. Comparison between group differences revealed that treatment group had significantly more improvement of KOOS pain (23.9±18.3 vs 7.2±15.9, p=0.03) and WOMAC pain (-5.0±3.6 vs -0.1±5.5, p=0.02) at 9 months compared with control group. However, the superiority was not sustained until 12 months. In comparison of percentage change from baseline, total KOOS in treatment group had improved significantly compared with control group at 3 and 9 months. The analysis of knee cartilage using MR T2 mapping revealed that the treatment group exhibited a less sharply increase in mean T2 value compared to the control group. Specifically, the mean difference of T2 values between baseline and 12 months was found to be significantly different between treatment group and control group (2.7 ± 13.2 and 5.1 ± 12.8, respectively. p=0.009). Nine patients of treatment group experienced the acute knee pain and swelling relieved by medical treatment, but there were no serious adverse events in all patients.
Conclusion: An intra-articular injection of allogenic BM-MSCs provided satisfactory pain relief for patients with knee OA compared to control group at 9 months’ follow-up. Also, comparison of T2 values from MR T2 mapping for cartilage between two groups showed that IA BM-MSCs could have a preventive effect of OA progression. Further studies with larger sample size, repetitive injections, and long-term MR follow-up are required.
To cite this abstract in AMA style:
Yang J, Lee B, Lee J, Ju J. Therapeutic Efficacy of Intra Articular Injection of Human Bone Marrow Derived Mesenchymal Stem Cells in Knee Osteoarthritis; Randomized, Double-blind, Placebo-controlled Clinical Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/therapeutic-efficacy-of-intra-articular-injection-of-human-bone-marrow-derived-mesenchymal-stem-cells-in-knee-osteoarthritis-randomized-double-blind-placebo-controlled-clinical-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/therapeutic-efficacy-of-intra-articular-injection-of-human-bone-marrow-derived-mesenchymal-stem-cells-in-knee-osteoarthritis-randomized-double-blind-placebo-controlled-clinical-trial/