Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Oral Chinese patent medicine (CPM) has been deemed to have analgesic and anti-inflammatory effects and is widely used as the first-line treatment for osteoarthritis (OA) in Asia countries. Despite this enthusiastic use of CPM for knee OA, its clinical efficacy remains unclear. We conducted an updated review to determine the effects of these therapies to inform clinical practice.
Methods: We performed a comprehensive search on PubMed, Cochrane Library, EMBASE, Biomedical Databases, National Knowledge Infrastructure, Wanfang, and VIP through May 2021. We included randomized controlled trials (RCTs) of oral CPM therapies as the first-line treatment in adults with knee OA. Knee OA was defined by the American College of Rheumatology criteria or the Chinese orthopedic association criteria. We also accepted the composite outcome criteria developed by the Chinese National Institute of Rheumatology as an endpoint (total effective rate, between 0-100%, higher score = better outcome), which assesses the overall pain, physical function and wellness. We extracted information on study characteristics, population characteristics, type, duration, frequency of interventions, and outcomes. Study quality was assessed in RevMan5.3 software using the Cochrane Risk of Bias Tool. The differences between treatment groups were reported as mean change or percentage improvement (P-value).
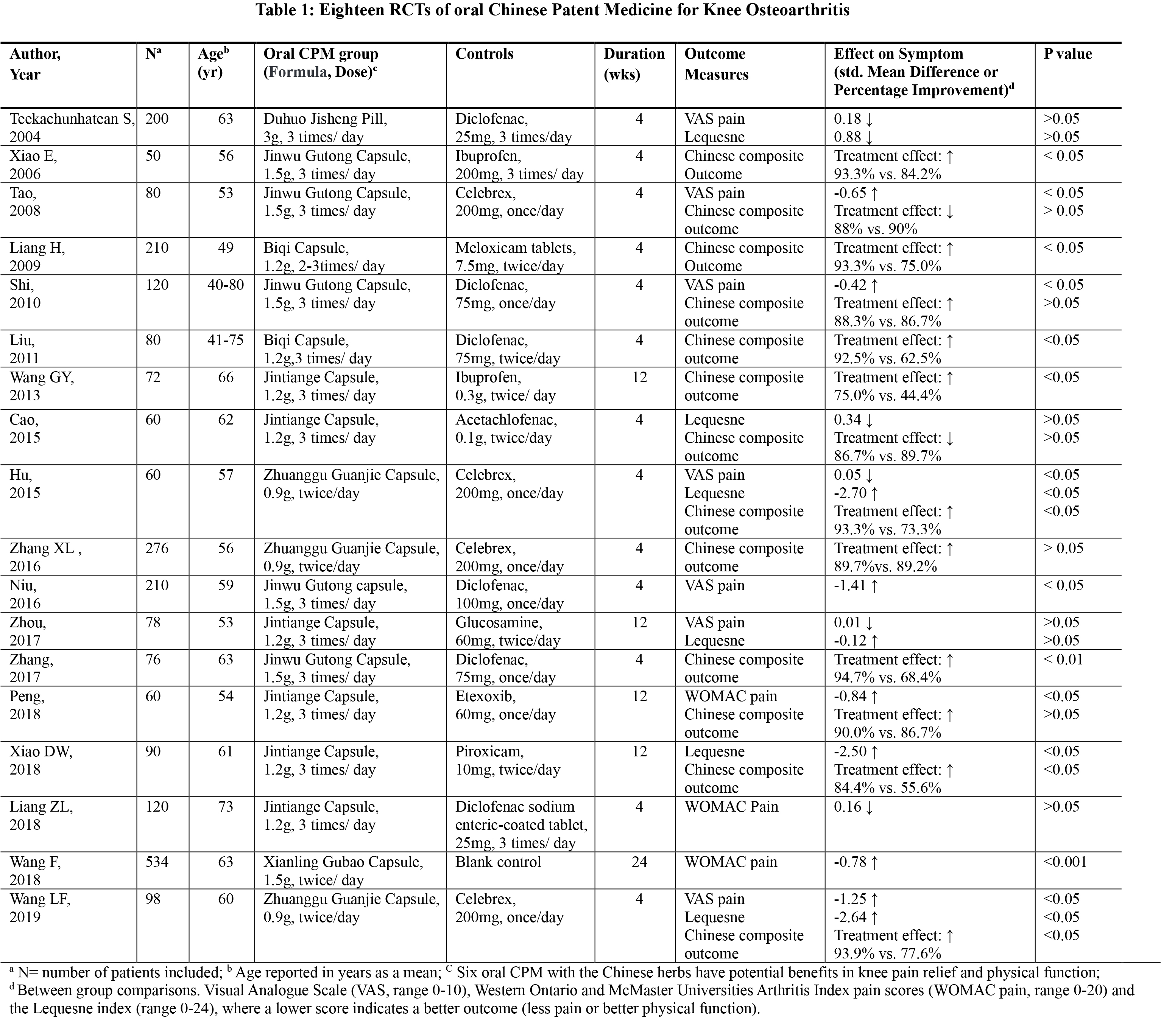
Results: We identified 1096 potentially relevant studies. Eighteen with a total of 2474 subjects (59% female, mean age = 59 years, mean pain duration = 3 years) met eligibility criteria. Seventeen studies were conducted in China and one in Thailand. Table 1 summarized the eighteen RCTs concerned with the effectiveness of oral CPMs for knee OA. Six types of oral CPMs including Jintiange Capsule, Jinwu Gutong Capsule, Zhuanggu Guanjie Capsule, Biqi Capsule, Xianling Gubao Capsule and Duhuo Jisheng Pill were prescribed as the first-line treatment based on the syndrome differentiation. The treatment varied duration from one to three times per day and lengths of the treatment ranged from 4 to 24 weeks. The control group treatments included oral non-steroidal anti-inflammatory drugs (17 studies) and placebo (1 study). Ten studies showed significant pain relief compared to the controls (std, Mean Difference [SMD] = -0.50; 95% CI, -0.86 to -0.13; p< 0.05). Thirteen studies reported a significant improvement in overall clinical symptoms on both pain and function (RR = 1.15; 95% CI, 1.09 to1.22; p< 0.00001). There were no significant differences in Lequesne index between the groups in six studies (MD = -1.11; 95% CI, -2.57 to 0.34; p=0.13). No serious adverse events occurred. Constipation was the most common adverse reaction in the oral CPM group, followed by dizziness, nausea, stomach discomfort and rash, and the incidence of adverse events was significantly lower than the controls.
Conclusion: This review showed promising results for oral CPM that is a safe and potential beneficial effects for knee OA. Further high-quality randomized controlled trials with longer follow-up periods are warranted.
To cite this abstract in AMA style:
Chen W, Song M, Jia Y, Sun J, Zhao Y, Xue Z, Chen Q, Zeng x, Wang C. Therapeutic Effects of Oral Chinese Patent Medicine on Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/therapeutic-effects-of-oral-chinese-patent-medicine-on-knee-osteoarthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/therapeutic-effects-of-oral-chinese-patent-medicine-on-knee-osteoarthritis/