Session Information
Session Type: Abstract Session
Session Time: 4:30PM-4:45PM
Background/Purpose: ORAL Surveillance (NCT02092467) was a randomized, open-label, non-inferiority, Phase 3b/4 study that assessed the relative risk of major adverse cardiovascular (CV) events (MACE) and malignancies with tofacitinib 5 and 10 mg twice daily (BID) vs TNF inhibitors (TNFi) in patients (pts) with active, moderate to severe RA, who had an inadequate response to MTX and a high risk of MACE (age ≥ 50 yrs; ≥ 1 additional CV risk factor). This analysis assessed the risk of venous thromboembolic events (VTE; including deep vein thrombosis [DVT] and pulmonary embolism [PE]) in ORAL Surveillance.
Methods: Pts were randomized 1:1:1 to receive tofacitinib 5 or 10 mg BID or a TNFi (etanercept 50 mg every week or adalimumab 40 mg every 2 weeks) with background MTX. A 2019 review by the Tofacitinib Rheumatology Data Safety Monitoring Board observed a statistically and clinically important difference in the occurrence of PE within the tofacitinib 10 mg BID treatment arm, compared with the TNFi control arm, resulting in a dose switch from tofacitinib 10 to 5 mg BID for the rest of the trial. All data are reported per randomized treatment. Incidence rates (IRs; pts with events/100 pt-yrs [PY]) were reported for adjudicated VTEs, DVT, and PE, and nominal p values comparing treatment arms were generated post hoc. The probability of not having an adjudicated event was assessed by Kaplan-Meier plots. Numbers Needed to Harm (NNH) were calculated post hoc. Multivariate Cox regression models were used post hoc to identify overall independent baseline (BL) risk factors for PE using backward model selection.
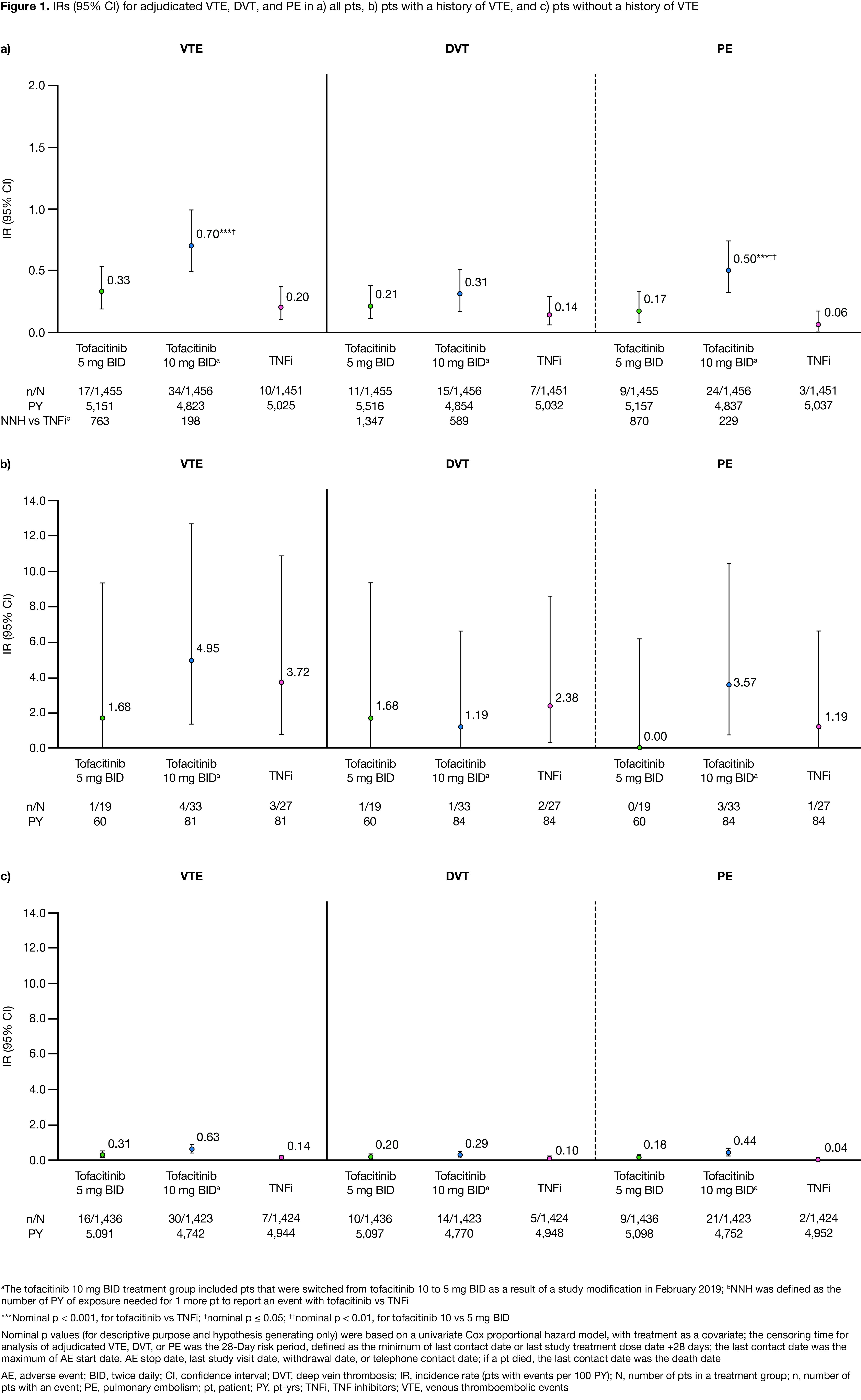
Results: This analysis included 1,455, 1,456, and 1,451 pts receiving tofacitinib 5 mg BID, 10 mg BID, and TNFi, respectively; BL pt characteristics were similar across treatment groups, with high mean disease activity (Clinical Disease Activity Index score, 39.7–39.9) and mean/median age of 61.2/60.0 yrs. In pts with VTE (n=66), mean age was 62.9–66.5 yrs across treatment groups. IRs for VTE, DVT, and PE were < 1.0 across treatment groups (Figure 1a); VTE, DVT, and PE IRs and probability of event were higher with tofacitinib 10 vs 5 mg BID, and higher for both tofacitinib doses vs TNFi (Figure 1a, Figure 2). NNH for tofacitinib 5 and 10 mg BID, respectively, vs TNFi were 763 and 198 PY for VTE, 1,347 and 589 PY for DVT, and 870 and 229 PY for PE. Across treatment groups, VTE, DVT, and PE IRs were higher in pts with vs without a history of VTEs (Figure 1b–c). Identified overall independent risk factors for PE across treatment groups included history of VTE; BL use of oral contraceptives or HRT; BL BMI ≥ 30 kg/m2; age ≥ 65 yrs; and history of hypertension (Figure 3).
Conclusion: VTE, DVT, and PE IRs were higher for tofacitinib (10 > 5 mg BID) vs TNFi, and were < 1.0 across treatment groups. IRs were generally consistent with ranges reported for tofacitinib and biologic DMARDs among pts at a high risk of a CV event;1 PE IR with tofacitinib 10 mg BID in ORAL Surveillance was higher than those reported in tofacitinib or biologic DMARD clinical trial or registry data.1
1. Mease P et al. Ann Rheum Dis 2020; 79: 1400-13
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by AG McCluskey, CMC Connect, funded by Pfizer Inc.
To cite this abstract in AMA style:
Charles-Schoeman C, Fleischmann R, Mysler E, Greenwald M, Wang C, Chen A, Connell C, Woolcott J, Menon S, Chen Y, Lee K, Szekanecz Z. The Risk of Venous Thromboembolic Events in Patients with RA Aged ≥ 50 Years with ≥ 1 Cardiovascular Risk Factor: Results from a Phase 3b/4 Randomized Safety Study of Tofacitinib vs TNF Inhibitors [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-risk-of-venous-thromboembolic-events-in-patients-with-ra-aged-%e2%89%a5-50-years-with-%e2%89%a5-1-cardiovascular-risk-factor-results-from-a-phase-3b-4-randomized-safety-study-of-tofacitinib-vs-tn/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-risk-of-venous-thromboembolic-events-in-patients-with-ra-aged-%e2%89%a5-50-years-with-%e2%89%a5-1-cardiovascular-risk-factor-results-from-a-phase-3b-4-randomized-safety-study-of-tofacitinib-vs-tn/