Session Information
Date: Saturday, November 12, 2022
Title: Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: There is an unmet medical need for the treatment of patients with active ankylosing spondylitis (AS) who have an inadequate response to NSAIDs. Prior network meta-analysis (NMA) did not include newer molecules under investigation, such as combined IL17A-IL17F inhibitors and Janus Kinase (JAK) inhibitors which have shown efficacy in previous clinical trials.
Due to the lack of head-to-head studies directly comparing the available therapies as well as the emergent therapies, this study aims to compare the relative efficacy of biologic and targeted synthetic DMARDs for the treatment of active AS.
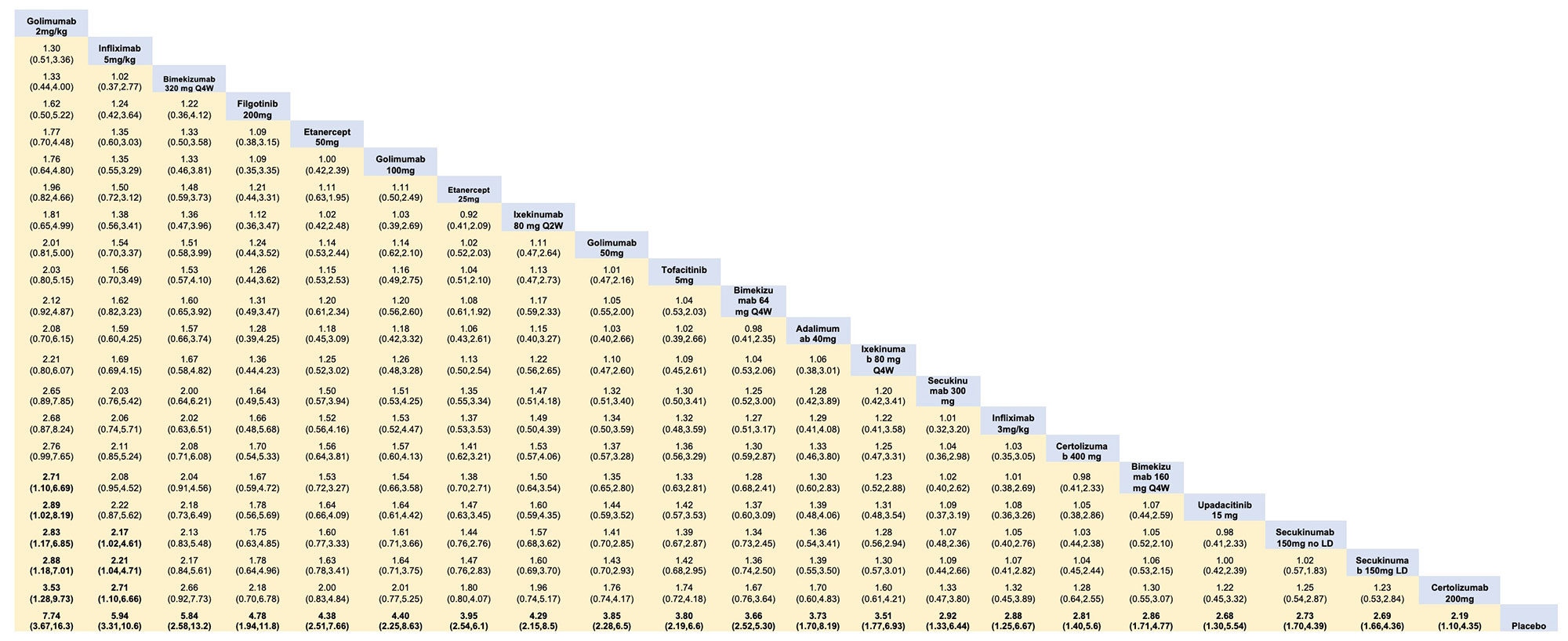
Methods: We conducted a Bayesian NMA of randomized clinical trials (RCTs) examining the relative efficacy of biologic and targeted synthetic DMARDs in patients with active AS who had inadequate response or intolerance to NSAIDs. In studies of IL 17A inhibitors, data of patients showing inadequate response to TNF inhibitors were excluded after analysis showed inconsistency likely due to high placebo responses. The systematic review was performed using PubMed, EMBASE, and Cochrane databases until May 2022. NMA was conducted by Stata 17.0 MP software using odds ratio (OR) with 95% credible interval (CrI) to assess the clinical effectiveness. Surface Under Cumulative Ranking curve (SUCRA) was used to analyze the relative efficacy ranking of different treatments in terms of achievement of ≥20% in the Assessment of Spondyloarthritis International Society Criteria (ASAS20) at 12-16 weeks.
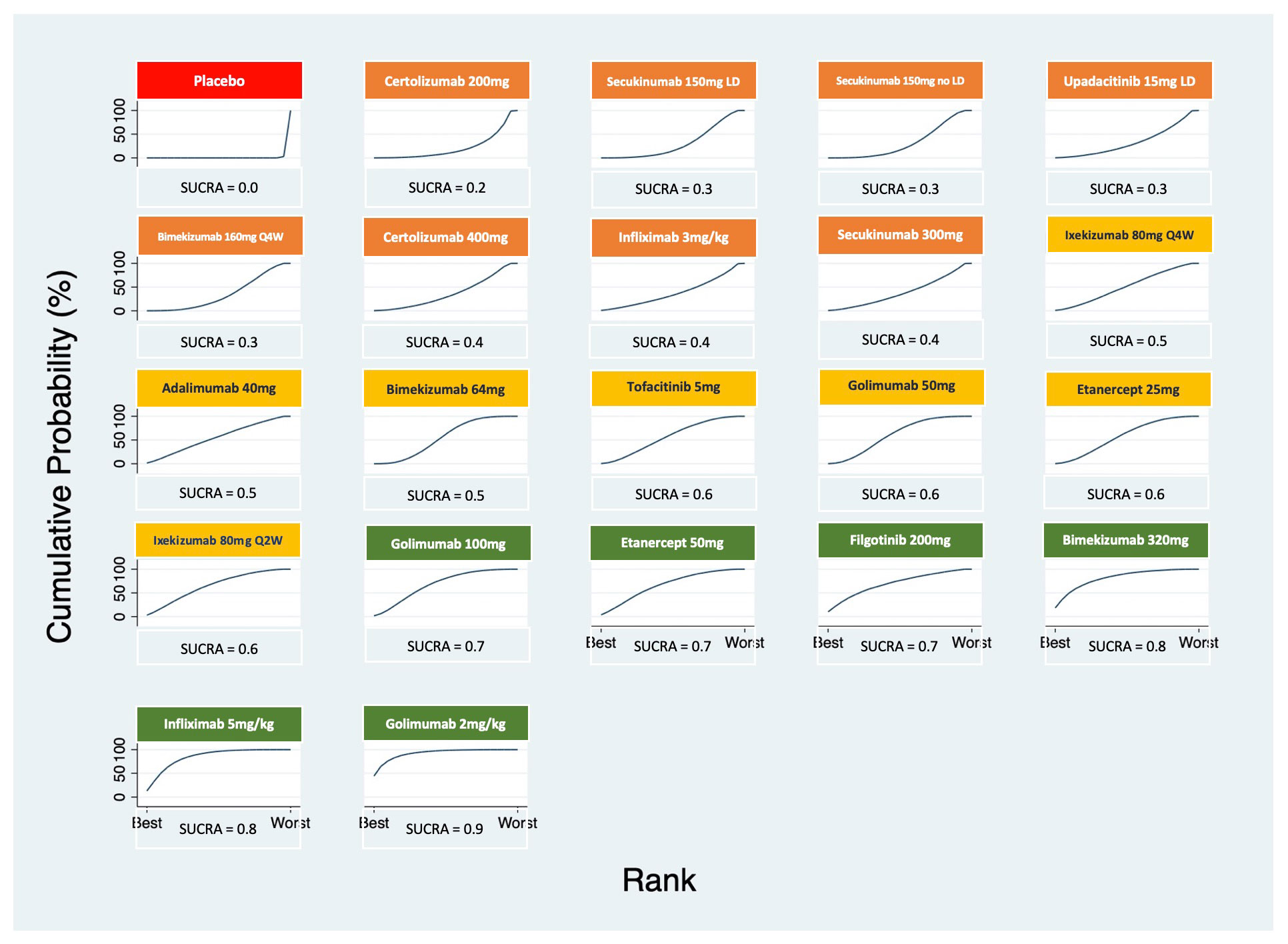
Results: We included 27 RCTs that enrolled 5,497 patients with active AS. There were 253 pairwise comparisons, including 32 direct comparisons of 22 interventions. All the interventions showed an improvement in ASAS20 response rate versus placebo (Table 1). Therapies that showed the highest response rate were the intravenous (IV) golimumab 2mg/kg (OR 7.74, 95% CrI 3.67- 16.33) and infliximab 5mg/kg (OR 5.94, 95% CrI 3.31 – 10.63). Apart from the IV formulations, there were no differences between therapies in achieving ASAS20; among these bimekizumab 320 mg (OR 5.84, 95% CrI 2.58 – 13.2) and filgotinib 200 mg (OR 4.78, 95% CrI 2.58-8.63) showed the highest response rates verus placebo. The ranking probability based on the SUCRA indicated that golimumab IV 2mg/kg (SUCRA = 0.9), infliximab IV 5mg/kg (SUCRA = 0.8) and bimekizumab 320 mg (SUCRA = 0.8) had the highest probability of achieving the best outcome (Figure 1). We arbitrarily ranked the best therapies, based on SUCRA cut-offs, as optimal (SUCRA ≥0.7), good (SUCRA 0.6-0.5), and effective (SUCRA ≤0.4) (Figure 1). The comparisons involving filgotinib, and upadacitinib were limited in the number of participants and, thus, may have been underpowered to detect statistical significance.
Conclusion: In patients with active AS, two approved therapies (IV golimumab and infliximab) were most efficacious in achieving ASAS20. Interestingly, two investigational therapies (bimekizumab 320 mg and filgotinib 200mg) seemed to be a convenient and efficacious alternative for which larger clinical trials are warranted.
Ref.
1. Castro A, et al. Arthritis Rheumatol.2020;72 (suppl 10). ABSTRACT NUMBER 1537
To cite this abstract in AMA style:
Diaz J, Quiceno G, Lee Ching C, Castro A. The Relative Efficacy of Biologic and Targeted Synthetic DMARDs in Ankylosing Spondylitis: A Network Meta-Analysis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/the-relative-efficacy-of-biologic-and-targeted-synthetic-dmards-in-ankylosing-spondylitis-a-network-meta-analysis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-relative-efficacy-of-biologic-and-targeted-synthetic-dmards-in-ankylosing-spondylitis-a-network-meta-analysis/