Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Historically, Heath Related Quality of Life (HRQL) in Sjogren’s Disease (SjD) has been measured by the EULAR Sjogren’s Syndrome Patient Reported Index (ESSPRI), which lacks important life impact domains. The Patient Reported Outcome Measurement Information System (PROMIS) offers a wider array of HRQL assessments, which were administered to patients alongside the Routine Assessment of Patient Index Data-3 (RAPID-3), a disability measure given to all patients during triage. Our objective was to evaluate the relationship between ESSPRI, PROMIS domains, and SjD disease activity with the hypothesis that there would be moderate positive correlation between PROs and disease activity.
Methods: A prospective study was conducted from November 2022 to September 2023 at a large academic medical center and gathered disease characteristic, disease activity, and patient reported outcome (PRO) data. PROs (ESSPRI, PROMIS-29, RAPID-3) were automatically assigned to SjD patients before their clinic visit and completed via the Epic patient portal. ACR/EULAR 2016 SJD criteria and the EULAR Sjogren’s Syndrome Disease Activity Index (ESSDAI) were completed and documented by rheumatologists via Epic smartforms. Mean demographics, disease activity, and HRQL scores were calculated for the first visit within the 6-month study period as well as Spearman and partial correlations to evaluate construct validity.
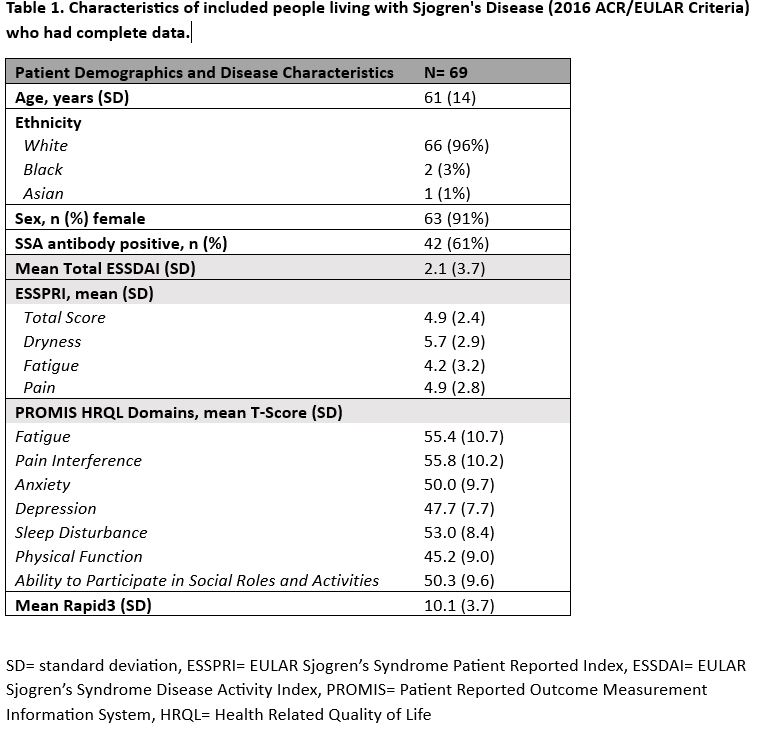
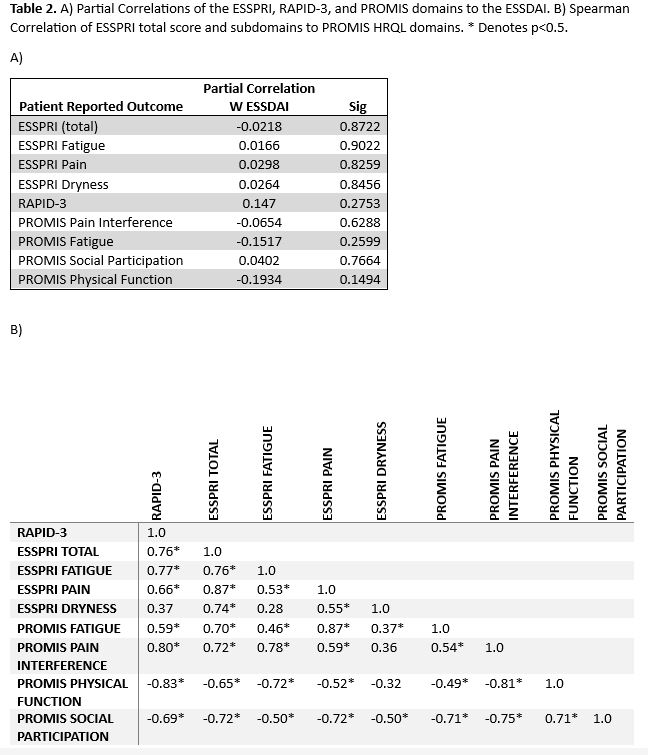
Results: There were 729/956 (76%) completed ESSPRIs during the study period collected from 594 unique patients. Of those, 197 patients met ACR/EULAR SjD criteria (33%) and had partially completed PROs/ESSDAI and 69 patients had fully completed PROs/ESSDAI. Patients had a mean (SD) age of 61 (14), were mostly female (91%) and white (96%). Mean (SD) ESSDAI scores were 2.1 (3.7) indicating low SjD disease activity with the most active subdomain being biological (n=10 (14%)). Mean (SD) ESSPRI scores were 4.9 (2.4) and considered as borderline-high symptom burden (≥5). There was moderate disability present (mean (SD) RAPID-3, 10.1 (3.7). Mean (SD) PROMIS pain interference and fatigue were greater than one-half SD higher than US population normative values (Table 1). Mood disturbance was not present as reflected by normal anxiety and depression mean T-scores. The total mean ESSPRI score and each of its domains (dryness, fatigue, pain) as well as PROMIS pain interference, fatigue, social participation, and physical function all poorly correlated with the mean ESSDAI score (Table 2A). ESSPRI fatigue displayed better positive correlation with PROMIS pain interference than PROMIS fatigue and vice versa (Table 2B). The ESSPRI total score had strong positive correlation with the RAPID-3, PROMIS pain interference, and fatigue and strong negative correlation with PROMIS social participation. ESSPRI dryness had low/moderate correlation with other HRQL domains.
Conclusion: The ESSDAI lacked significant correlation to all PROs tested suggesting that patient symptomatology is complex and not solely connected to SjD disease activity. Contrary to expected, there was only moderate positive correlation for PROMIS pain interference and ESSPRI pain and PROMIS fatigue and ESSPRI fatigue.
To cite this abstract in AMA style:
DiRenzo D, Johr C, Sandorfi N, McCoy S, Grader-Beck T. The Relationship of the ESSDAI to Health-Related Quality of Life in People Living with Sjogren’s Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-relationship-of-the-essdai-to-health-related-quality-of-life-in-people-living-with-sjogrens-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-relationship-of-the-essdai-to-health-related-quality-of-life-in-people-living-with-sjogrens-disease/