Session Information
Date: Monday, November 13, 2023
Title: (1221–1255) Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Juvenile systemic sclerosis (jSSc) is an orphan disease with a prevalence of 3 in 1,000,000 children. Currently no medications are licensed for the treatment of jSSc. Due to its rarity, only recently the first management and treatment guidelines have been published, the jSSc SHARE (Single Hub and Access point for paediatric Rheumatology in Europe) recommendations, reflecting consensus opinion upon pediatric rheumatologists(1). We reviewed the applied medication in the treatment of the patients in the juvenile systemic scleroderma inception cohort (jSScC) up to April 2023.
Methods: We reviewed the change of the applied medication in the treatment of jSSc patients over 36 months in the jSScC. The frequency of medications was calculated across the cohort at timepoint 0 (enrollment), and 36 months. jSScC is a prospective cohort of jSSc patients, who developed the first non-Raynaud ́s symptom before the age of 16 years and are under the age of 18 years at the time of inclusion.
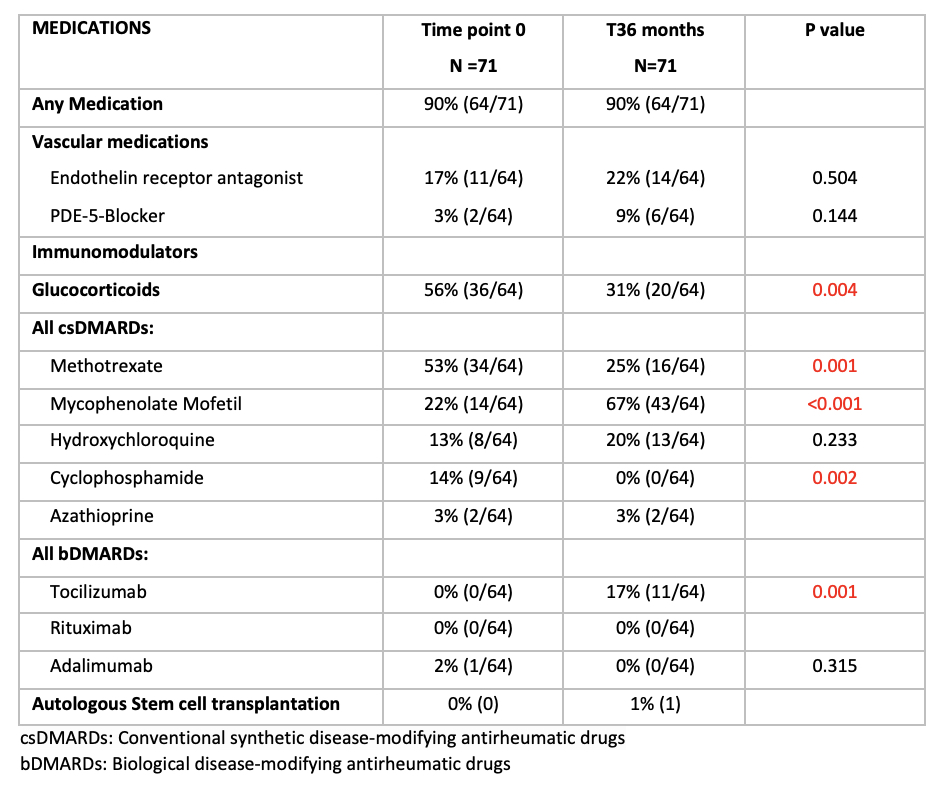
Results: We extracted data from 71 patients from the jSScC who were followed for 36 months, 75% had diffuse subtype. At the time of inclusion in the cohort the median disease duration was 2.4 years, median age of the first non-Raynaud symptom was 10.3 years. We captured the recorded medications at 0 months and 36 months. 64/71(90%) received any kind of Disease modifying drug (DMARD).
The glucocorticoid use decreased from month 0 to 36 months from 56% to 31% (p=0.004).The methotrexate use decreased from 53% to 25% (p=0.001), in opposite the mycophenolate use increased from 22% to 67% (p< 0.001). The cyclophosphamide use decreased from 14% to 0% (p=0.002). Tocilizumab use increased from 0% to 17% (p=0.001). All other medication use showed no significant changes. Endothelin receptor antagonist was used in 17% patients at time point 0 and 22% at 36 months. PDE-5 blocker use increased from 3% to 9%.
Conclusion: At baseline half of the patients were on glucocorticoids. This is more frequent than typical adult SSc practice but coincides with jSSc SHARE treatment recommendations (#1) (1). After 36 months observation in the cohort over 90% of patients received a DMARD therapy. Methotrexate and mycophenolate mofetil were the most commonly prescribed DMARDs, which also reflects the SHARE treatment recommendations (#2 and #3).At 36 months the use of glucocorticoids, methotrexate and cyclophosphamide decreased, and the use of mycophenolate and tocilizumab increased. In general, biological DMARDs are typically considered in severe or refractory disease (SHARE recommendation #7), reflecting the lower percentage compared to csDMARDs. Endothelial receptor antagonists, such as bosentan, were used over time in approximately 20% of the patients, reflecting SHARE recommendation #6 for pulmonary hypertension and/or digital tip ulcers. This is the first evaluation looking at clinical medication practice pattern in jSSc over 36 months, and its comparison to recently published consensus guidelines.
This project was supported by an unrestricted grant from “Joachim Herz Stiftung”
1 Foeldvari I, Culpo R, Sperotto F, Anton J, Avcin T, Baildam E, et al. Consensus-based recommendations for the management of juvenile systemic sclerosis. Rheumatology (Oxford). 2021;60(4):1651-8.
To cite this abstract in AMA style:
Foeldvari I, Klotsche J, Kasapcopur O, Adrovic A, Feldman B, Torok K, TErreri M, Sakamoto A, Anton J, Appenzeller S, Marrani E, Santos M, SZTAJNBOK F, Berntson L, Brunner J, Kostik M, Nuruzzaman F, Helmus N. The Pattern of Medication Use Significantly Changed over 36 Months Observation Period. Result from the Juvenile Scleroderma Inception Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-pattern-of-medication-use-significantly-changed-over-36-months-observation-period-result-from-the-juvenile-scleroderma-inception-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-pattern-of-medication-use-significantly-changed-over-36-months-observation-period-result-from-the-juvenile-scleroderma-inception-cohort/