Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Antiphospholipid syndrome (APS) is an acquired thrombo-inflammatory disease characterized by persistent antiphospholipid antibodies (aPL). APS patients experience significant morbidity and mortality, much of which can be attributed to cardiovascular complications. Indeed, APS patients have accelerated atherosclerosis at rates comparable to those with diabetes. A recent population-based study found that detection of IgA aPL in the general population is associated with a 4-fold increase in future atherosclerotic cardiovascular events. IgA aPL was also independently associated with impaired cholesterol efflux capacity. While research has traditionally focused on the pro-atherogenic role of IgG aPL, the impact of IgA aPL on accelerated atherosclerosis remains unclear. Here, we assessed HDL function and foam cell-forming capacity in primary APS/aPL-positive plasma. We also evaluated some potential mechanistic impacts of IgA anti-beta-2 glycoprotein I (aβ2GPI) on accelerated atherosclerosis.
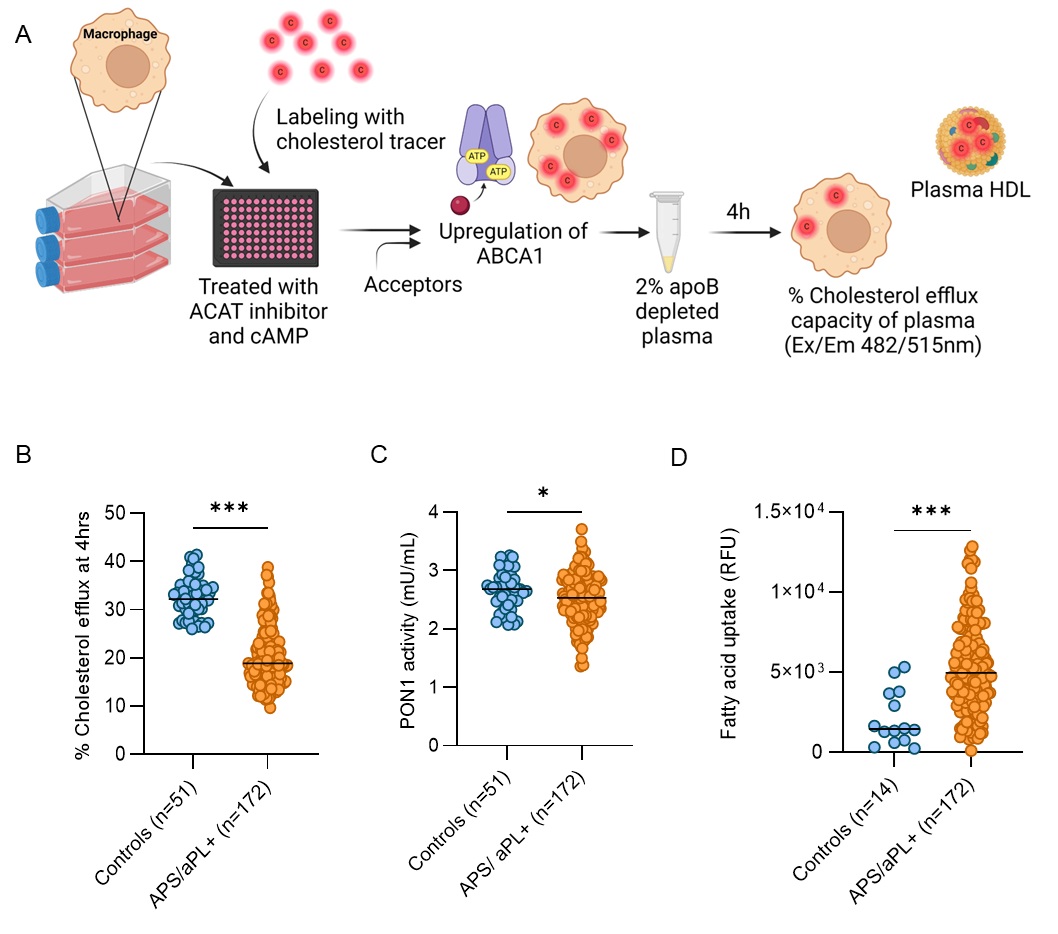
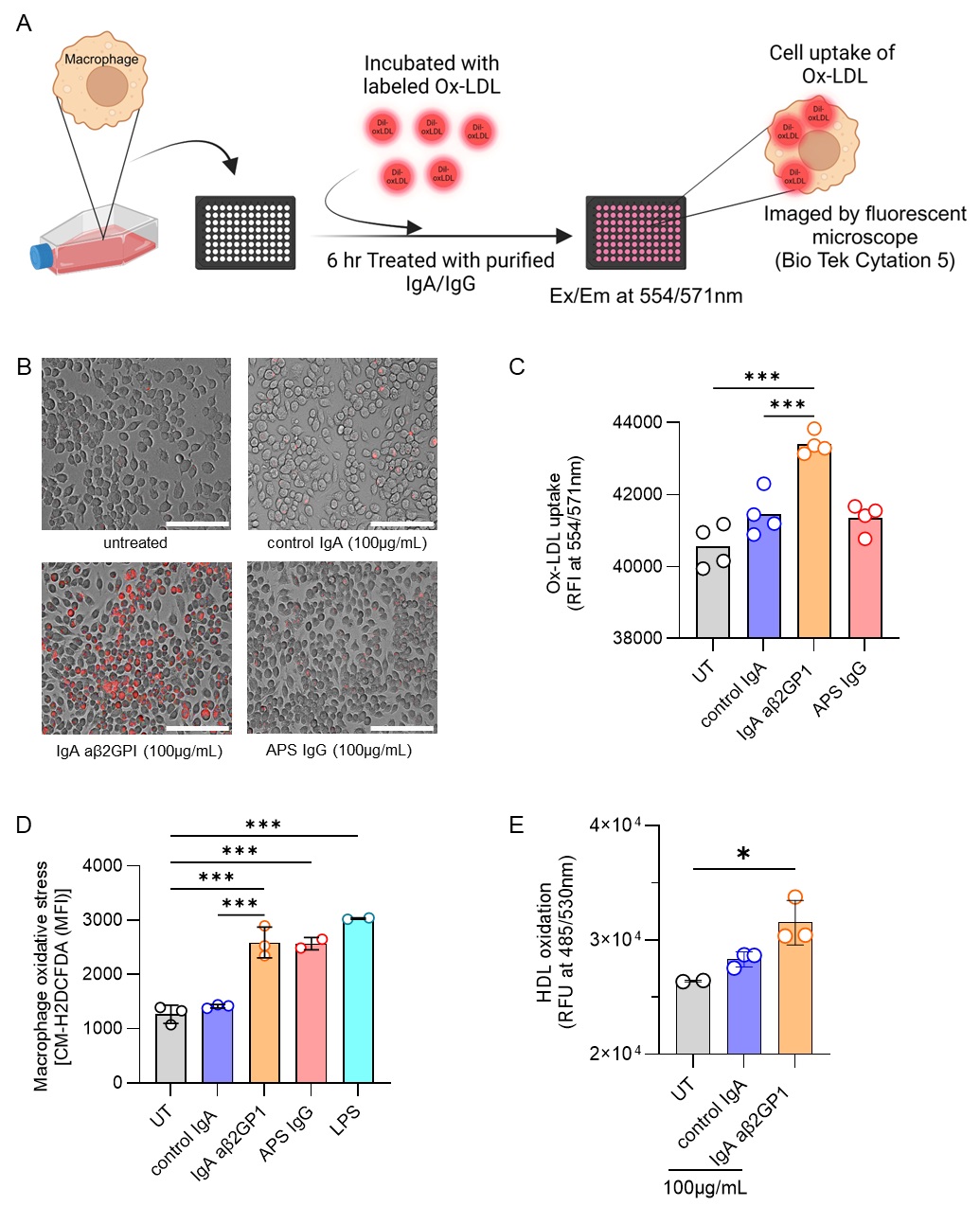
Methods: We evaluated the cholesterol efflux capacity (CEC, a measure of HDL function) in apoB-depleted plasma and antioxidant paraoxonase 1 (PON1) activity of serum from 172 primary APS/aPL-positive patients and 51 healthy controls. None of the studied individuals had lupus. We also asked whether the plasma favored fatty acid uptake in an assay of foam cell formation. Additionally, we investigated the impact of total IgA isolated from IgA aβ2GPI-positive patients on macrophage oxidative stress, foam cell formation, and HDL oxidation—key factors in atherogenic plaque development. To do this, we employed flow cytometry, fluorescent microscopy, and plate-based assays.
Results: Primary APS/aPL-positive plasma exhibited impaired CEC and reduced PON1 activity, both suggestive of dysfunctional HDL (Fig 1A-C). As expected, there was a positive correlation between CEC and PON1 activity (r=0.285, p< 0.001). Plasma from APS/aPL-positive patients also promoted fatty acid uptake by macrophages (Fig 1D), a key factor in pro-atherogenic foam cell formation. There was a negative correlation between fatty acid uptake and CEC (r=-0.206, p< 0.01), and plasma from patients with a history of thrombosis showed more fatty acid uptake than those without (p< 0.01). Mechanistically, total IgA isolated from IgA aβ2GPI-positive patients enhanced oxidized LDL uptake (Fig 2A-C) and triggered reactive oxygen species (ROS) production by macrophages (Fig 2D). Furthermore, IgA aβ2GPI potentiated HDL oxidation in a cell-free assay, likely counteracting HDL’s normally protective antioxidant properties (Fig 2E).
Conclusion: Our study reveals, for the first time, that primary APS/aPL-positive patients have significantly impaired HDL function. Further, IgA aβ2GPI can promote macrophage oxidative stress, accelerate foam cell formation, and directly impair HDL function. These findings provide insights into the pathogenesis of accelerated atherosclerosis in APS.
To cite this abstract in AMA style:
Sugur K, Chong E, Yalavarthi S, Kmetova K, Kluge L, Liang W, Sarosh C, Somanathapura K N, Madison J, Tambralli A, Knight J, Zuo Y. The Mechanistic Impact of IgA anti-beta-2 Glycoprotein I on Accelerated Atherosclerosis in Primary APS [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-mechanistic-impact-of-iga-anti-beta-2-glycoprotein-i-on-accelerated-atherosclerosis-in-primary-aps/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-mechanistic-impact-of-iga-anti-beta-2-glycoprotein-i-on-accelerated-atherosclerosis-in-primary-aps/