Session Information
Date: Friday, November 6, 2020
Title: Patient Outcomes, Preferences, & Attitudes Poster I: RA, Spondyloarthritis, & OA
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The importance of patient-relevant outcomes, such as pain, fatigue and physical functioning, has been long established in the field of rheumatoid arthritis (RA). Patient-reported outcomes (PROs) questionnaires evaluate these outcomes in RA clinical trials and can differentiate treatments. However, this ability depends on the interpretation of PRO endpoints. Measuring PRO changes that are meaningful to patients can help clinicians optimize the treatment selection and evaluate outcomes within the treat-to-target approach, recommended by ACR. For the incoming RA treatments within the Janus kinase inhibitors (JAKis) class, we aimed to evaluate the available metrics to capture meaningful individual-patient-level change in most common PROs.
Methods: A targeted PubMed literature search was conducted to identify RA clinical trials of JAKis published between 1st Jan 2009 and 1st April 2020 that included a PRO as an outcome. We reviewed relevant publications and conference abstracts to identify values used to interpret PROs. Follow-up literature searches were conducted to determine and critically appraise methods used to derive these values.
Results: Among 101 papers meeting the inclusion criteria, 35 reported the proportion of patients achieving a minimal (clinically) important difference [M(C)ID] on Health Assessment Questionnaire Disability Index (HAQ-DI), Pain visual analogue scale (VAS), Patient Global Assessment (PtGA), Short-Form 36 (SF-36), Functional Assessment of Chronic Illness–Fatigue (FACIT-Fatigue), Insomnia Severity Index (ISI), morning stiffness severity and duration. However, the secondary methodological search revealed no evidence supporting the application of these values to interpret individual-patient-level meaningful change (Table 1).
Conclusion: Despite common use of PROs MID/MCID thresholds in the RA clinical trials of JAKis, current methods lack evidence of the methodologically robust magnitude of change meaningful to individuals. These findings highlight a need to re-evaluate the patient-relevance of PRO metrics in RA clinical trials, and potentially explore other endpoints, such as the Patient Acceptable Symptom State. The availability of methodologically robust, patient-relevant, clinically-meaningful change thresholds for PROs can help determine most patient-relevant RA therapies.
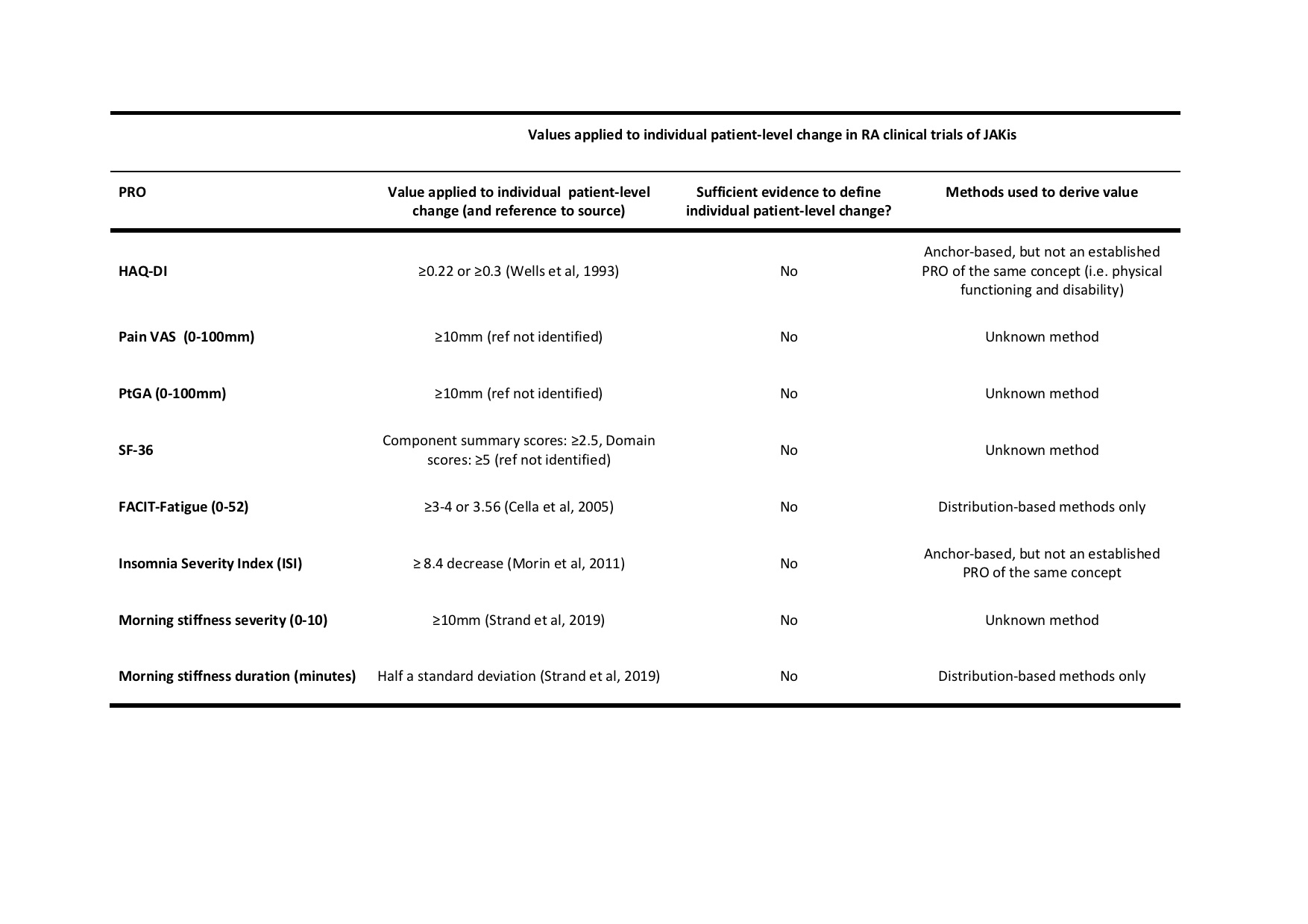 Table 1: Values used to interpret individual patient-level change on PROs in RA
Table 1: Values used to interpret individual patient-level change on PROs in RA
To cite this abstract in AMA style:
Taylor P, Dore R, Williams K, Acaster S, Antonova J, Genovese M. The Interpretation of Patient-reported Outcomes in Rheumatoid Arthritis: Do Clinical Trials Adequately Evaluate Meaningful Improvements for Patients? [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-interpretation-of-patient-reported-outcomes-in-rheumatoid-arthritis-do-clinical-trials-adequately-evaluate-meaningful-improvements-for-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-interpretation-of-patient-reported-outcomes-in-rheumatoid-arthritis-do-clinical-trials-adequately-evaluate-meaningful-improvements-for-patients/
