Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Lipid peroxidation is the result of disrupted redox homeostasis and functions as a key component in various cell death programs. Neutrophil extracellular trap (NET) release or NETosis is a specialized type of neutrophil cell death characterized by exuberant release of prothrombotic and proinflammatory tangles of chromatin, microbicidal proteins, and oxidative enzymes. In patients with APS, anti-beta-2 glycoprotein I antibodies engage the neutrophil surface and promote NETosis, thereby amplifying thromboinflammation. While increased lipid peroxidation has been described in APS, the intersection between lipid peroxidation and neutrophils remains unexplored. We aimed to dissect the impact of lipid peroxidation on APS-relevant NETosis and to determine the extent to which inhibitors of lipid peroxidation might be viable therapeutic strategies for APS.
Methods: Healthy donor neutrophils were treated for 4 hours with the classic NET stimulator phorbol 12-myristate 13-acetate (PMA), IgG purified from patients with APS (APS IgG), or IgG purified from heterologous healthy controls (control IgG). Lipid peroxidation was assessed by flow cytometry using BODIPY 581/591 C11 (2 µM), a fluorescent lipid peroxidation reporter molecule. NETosis was quantified by neutrophil elastase activity using a fluorogenic elastase substrate (0.5 µM). Tested NETosis blockers included the myeloperoxidase (MPO) inhibitor 4-aminobenzoic acid hydrazide (500 µM) and the peptidylarginine deiminase 4 (PAD4) inhibitor GSK484 (20 µM). Lipid peroxidation inhibitors included UAMC 3203 (1 µM), liproxstatin-1 (LPX) (5 µM), and a water-soluble analog of vitamin E (Trolox) (1 mM).
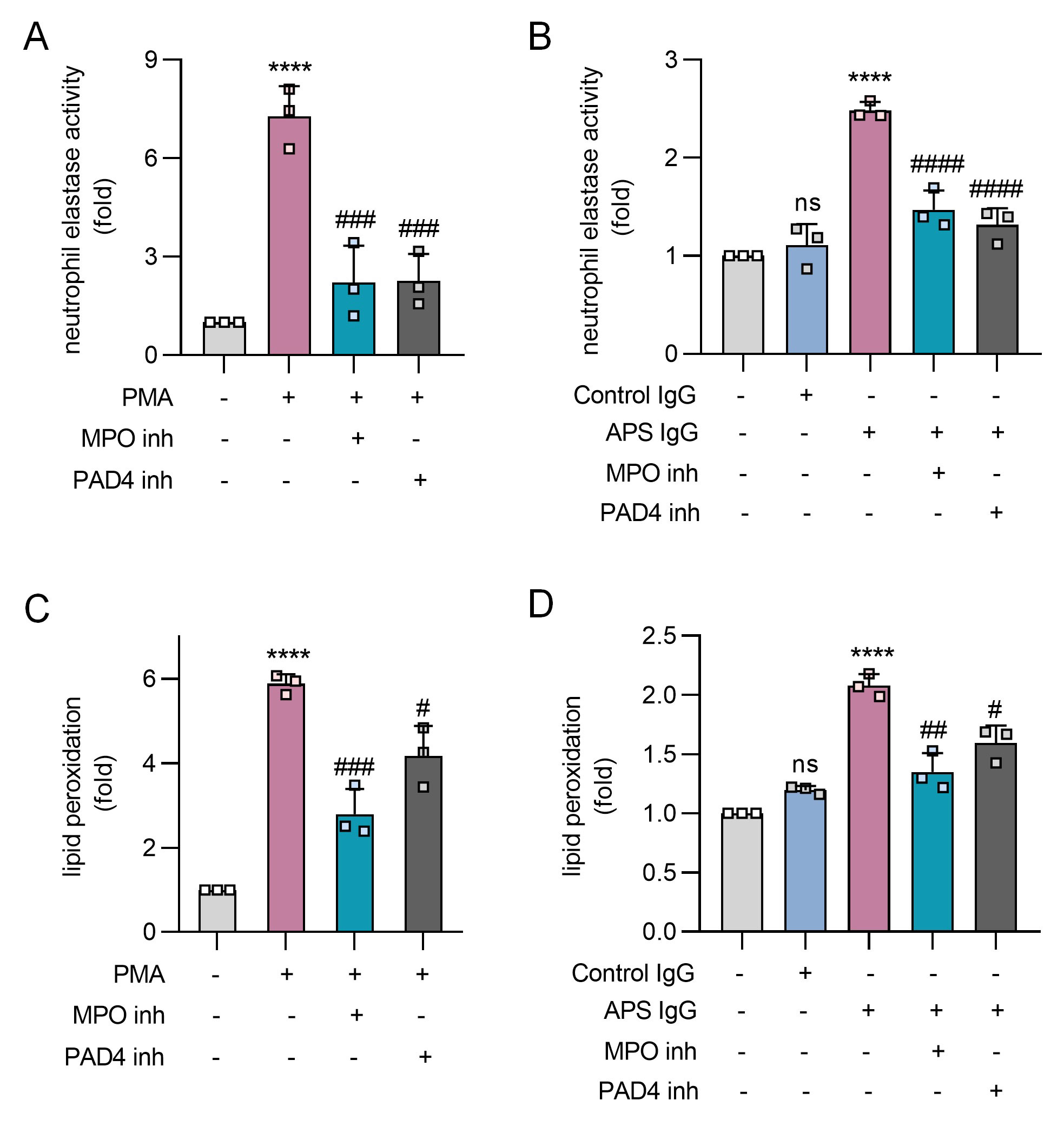
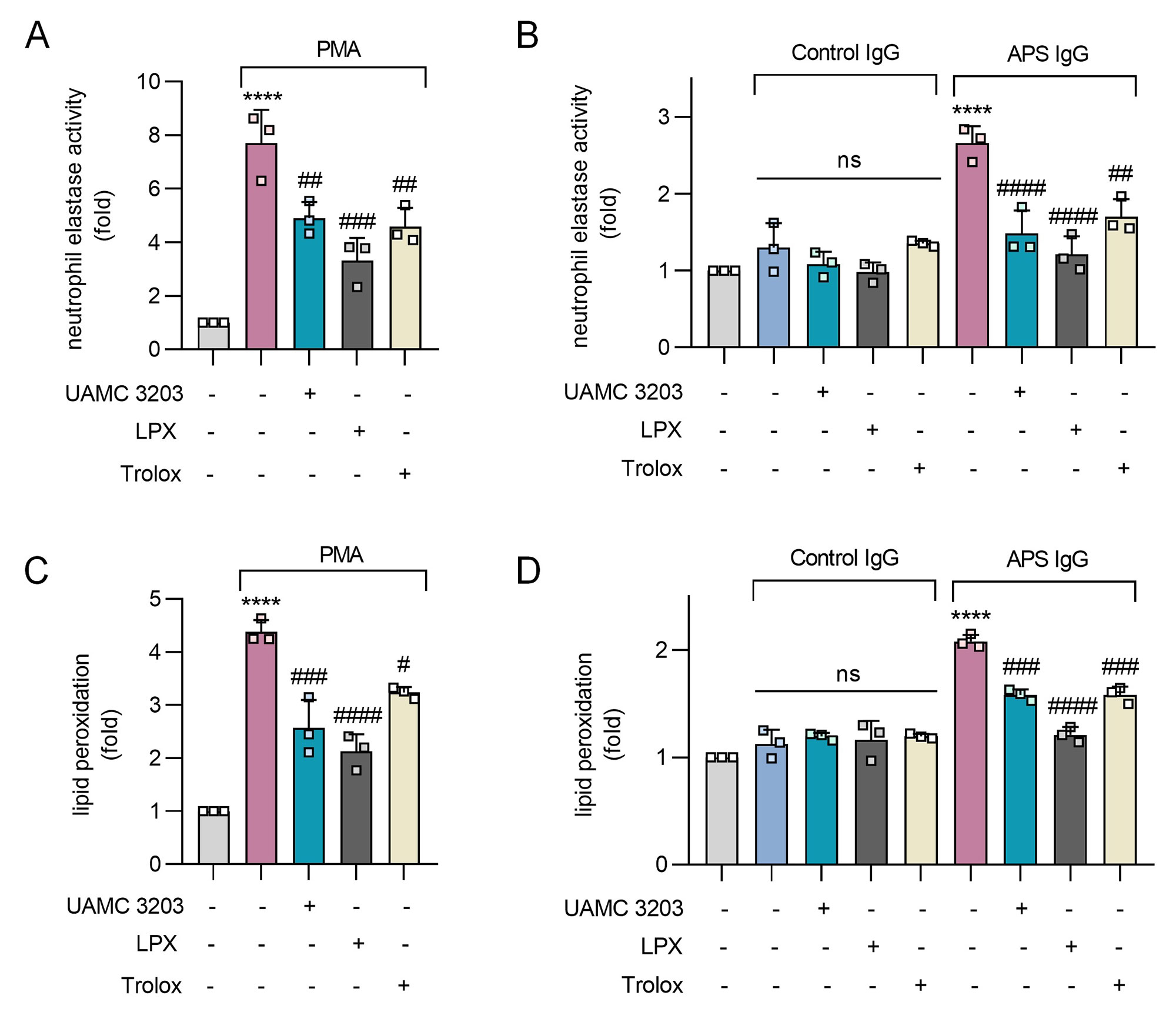
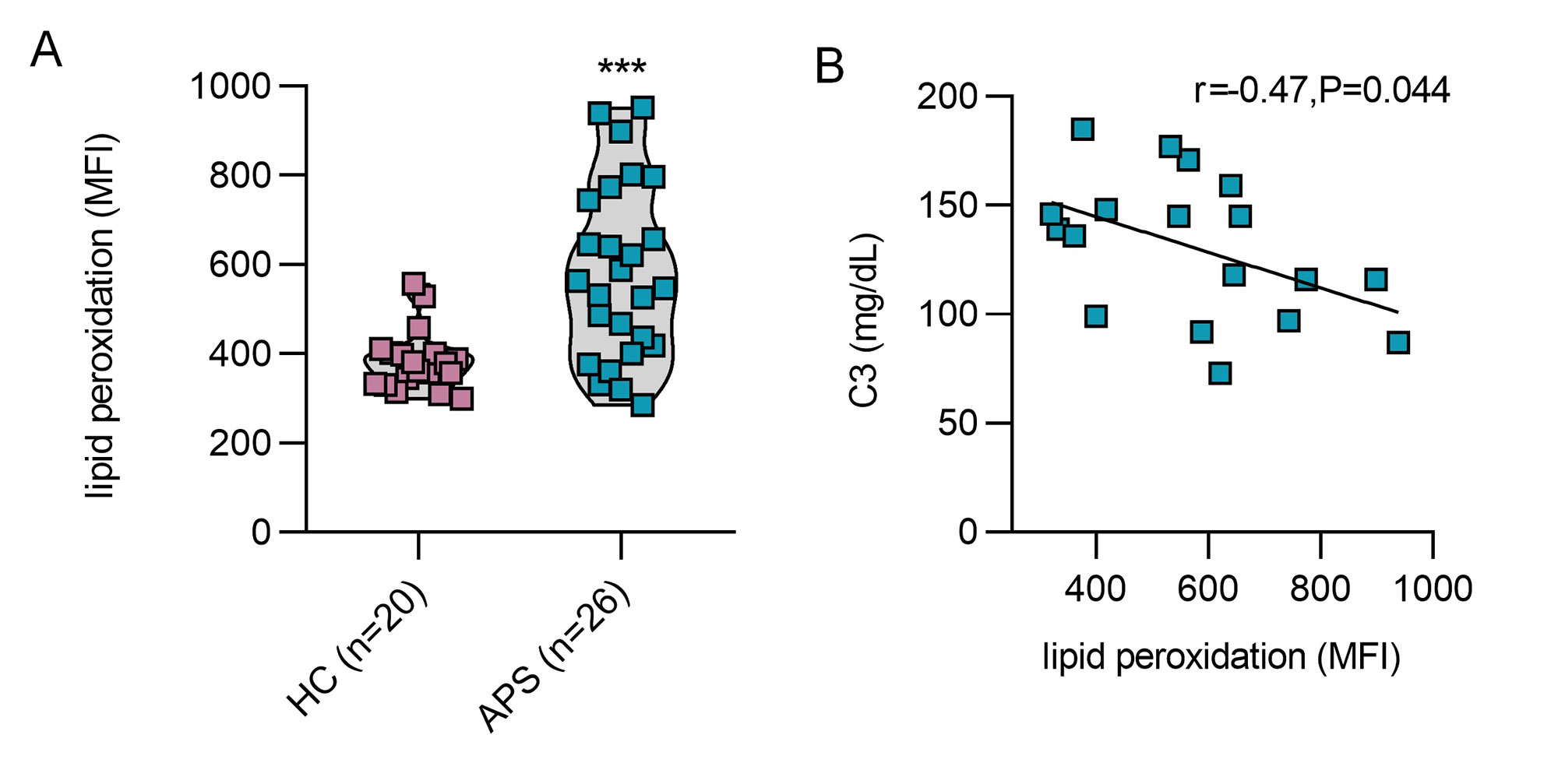
Results: PMA and APS IgG promoted not only NETosis (Fig 1A, 1B), but also marked lipid peroxidation of healthy donor neutrophils (Fig 1C, 1D). Both PMA- and APS IgG-mediated lipid peroxidation were prevented by inhibition of the key neutrophil oxidative catalyst MPO, as well as by inhibition of the NETosis regulatory enzyme PAD4 (Fig 1). When assessing the impact of traditional lipid peroxidation inhibitors on NETosis (Fig 2A, 2B), PMA- and APS IgG-mediated NETosis were suppressed by all three inhibitors (UAMC 3202, LPX, and Trolox). As expected, these inhibitors also potently suppressed neutrophil lipid peroxidation (Fig 2C, 2D). In primary aPL positive or APS patients (Fig 3A), lipid peroxidation of circulating neutrophils (n=26) was markedly higher than for healthy control neutrophils (n=20, p< 0.001). Interestingly, neutrophil lipid peroxidation negatively correlated with complement C3 level (r=-0.47, p=0.044) among these patients (Fig 3B).
Conclusion: In summary, these data suggest that MPO-mediated lipid peroxidation is required for NET formation in APS. Circulating neutrophils of primary aPL positive or APS patients demonstrate significantly accelerated lipid peroxidation, which could have implications for the association of beta-2-glycoprotein I with the neutrophil plasma membrane. Further studies are underway to evaluate lipid peroxidation inhibitors as a therapeutic strategy in APS.
To cite this abstract in AMA style:
S K N, Hoy C, Rysenga C, Yalavarthi S, Sarosh C, Navaz S, Madison J, Knight J, Zuo Y. The Interplay Between Lipid Peroxidation and Neutrophil Extracellular Trap (NET) Formation in APS Pathogenesis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/the-interplay-between-lipid-peroxidation-and-neutrophil-extracellular-trap-net-formation-in-aps-pathogenesis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-interplay-between-lipid-peroxidation-and-neutrophil-extracellular-trap-net-formation-in-aps-pathogenesis/