Session Information
Date: Sunday, November 13, 2022
Title: Abstracts: Spondyloarthritis Including PsA – Treatment II: PsA
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: While evidence in patients (pts) with RA suggests that switching to a therapy with a different mechanism of action (MOA) may be more effective than cycling among TNF inhibitors (TNFi) after discontinuing a 1st line TNFi, the relative effectiveness of cycling vs switching in pts with PsA is currently unknown. This study compared clinical outcomes between pts with PsA who initiated a TNFi vs a non-TNFi biologic after discontinuing a 1st line TNFi in a real-world setting.
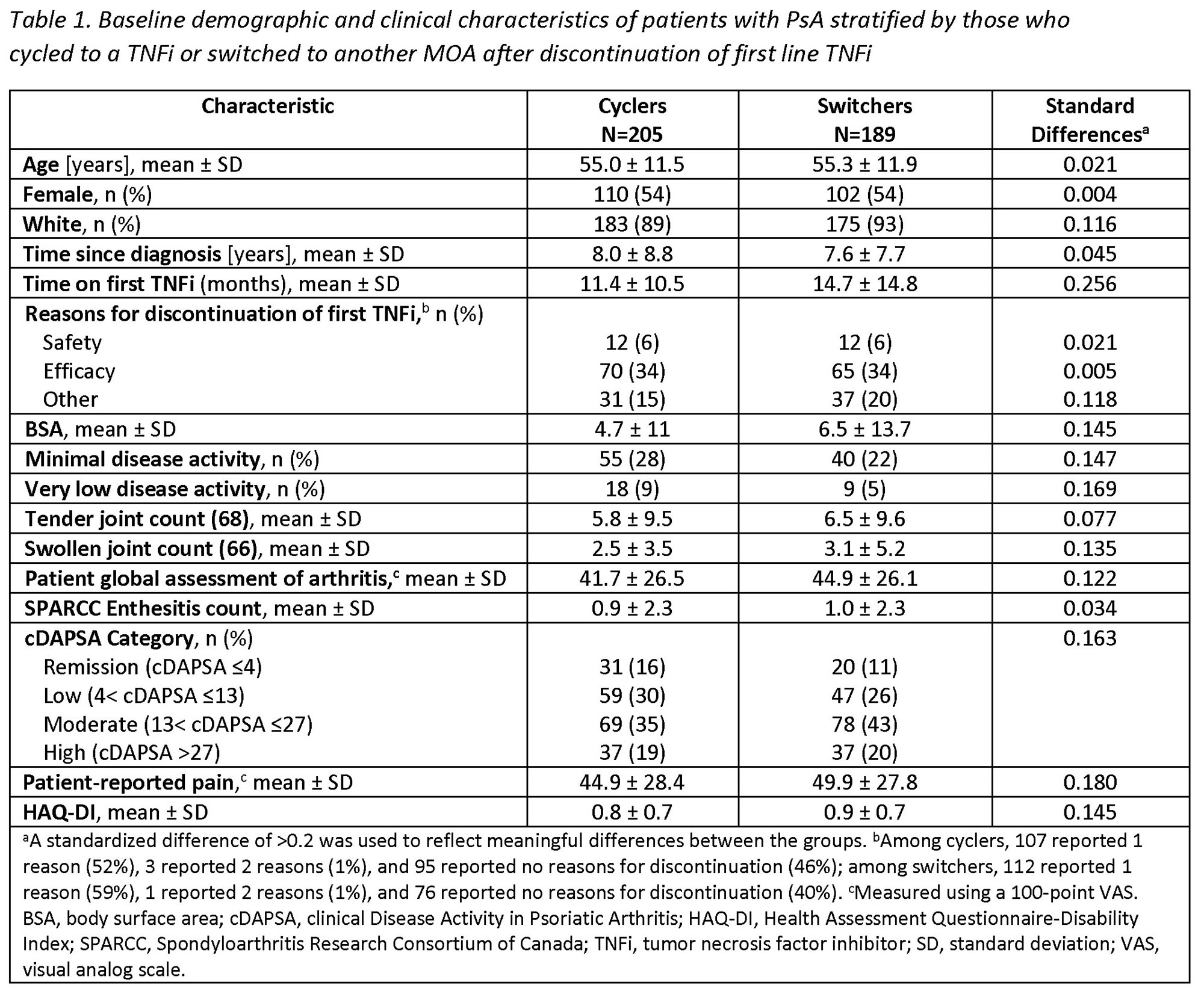
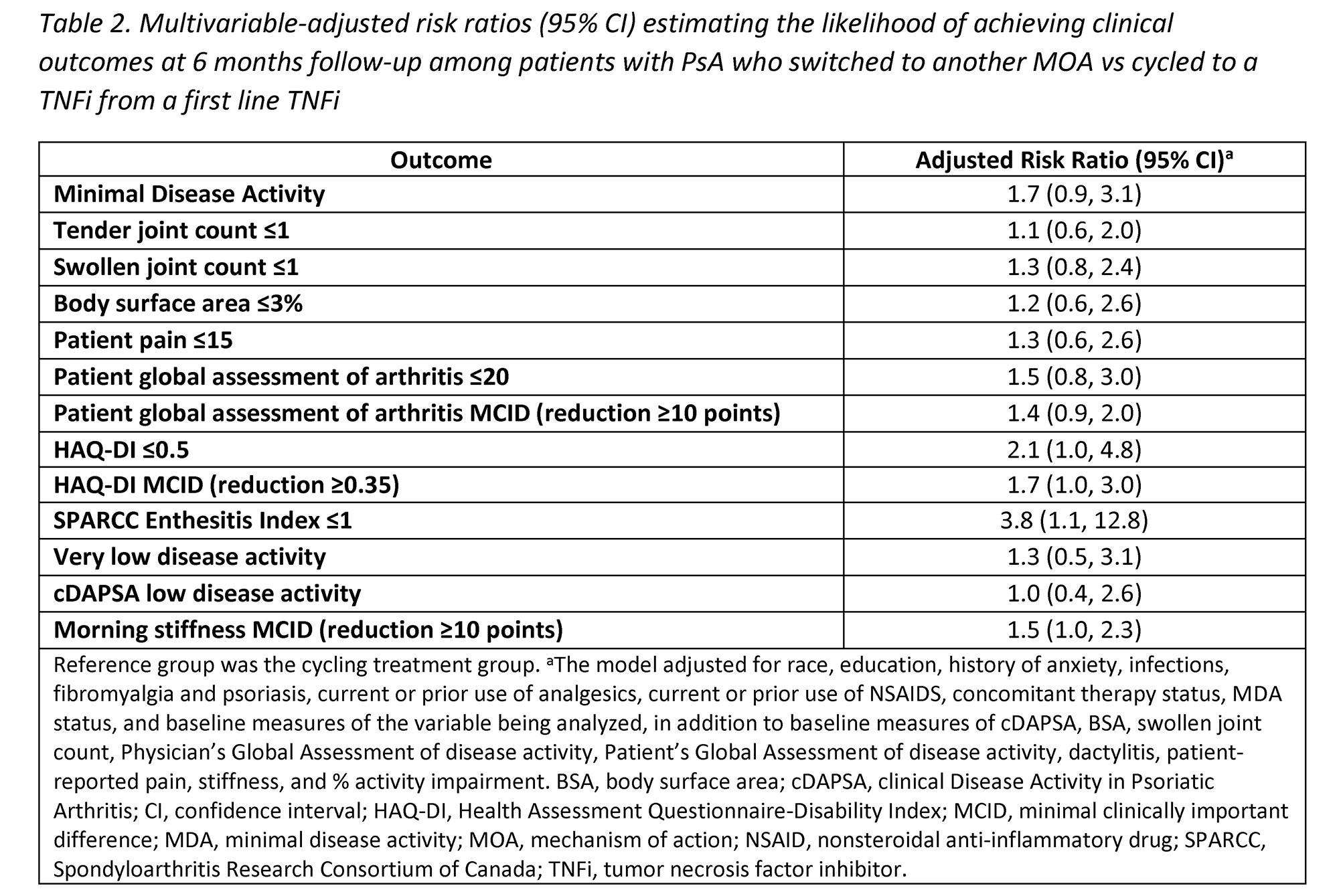
Methods: Pts with a clinical diagnosis of PsA enrolled in the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry who initiated a 2nd line advanced therapy (baseline) after discontinuation of 1st line TNFi between May 2013–January 2022 were included. Eligible pts had a follow-up visit 6-months after initiating the 2nd line therapy. Pts were stratified into two cohorts: 1) those who initiated a 2nd line TNFi (cyclers) and 2) those who initiated a 2nd line non-TNFi biologic (switchers). Baseline characteristics were summarized descriptively and compared between the groups using standardized differences (d; 0.2, 0.5, 0.8 considered small, medium, and large effects). Multivariable adjusted Poisson regression models were used to calculate risk ratios (RR) and 95% confidence intervals (CI) estimating the association between cycling vs switching status and clinical outcomes at 6 months.
Results: Among 394 eligible pts initiating a 2nd line therapy, there were 205 (52%) cyclers and 189 (48%) switchers. Groups were similar at baseline in mean age (55 years) and gender (54% female) (Table 1). Cyclers were on 1st line TNFi therapy for a shorter duration vs switchers (11.4 vs 14.7 months, d=0.26). At baseline, switchers had greater severity of psoriasis (mean body surface area; 6.5 vs 4.7) and worse disease activity, with lower proportions in a state of minimal disease activity (MDA; 22% vs 28%) or low disease activity (clinical disease activity in PsA [cDAPSA] LDA; 26% vs 30%) compared to cyclers, though differences were small (all d< 0.2). At the 6-month follow-up, switchers trended towards having an increased likelihood of achieving all clinical outcomes vs cyclers, though most CIs included 1.0 (Table 2). Data suggest switchers had 70% greater likelihood of achieving MDA (RR [95% CI] = 1.7 [0.9, 3.1]) and a nearly 4 times higher likelihood of achieving a Spondyloarthritis Research Consortium of Canada Enthesitis index score ≤1 (3.8 [1.1, 12.8]). Switchers vs cyclers had 2 times the likelihood of achieving HAQ-DI ≤0.5 (2.1 [1.0, 4.8]) and a 30% greater likelihood of achieving a pt pain score ≤15 (1.3 [0.6, 2.6]); similarly, switchers had a greater likelihood of achieving MCID in HAQ-DI (1.7 [1.0, 3.0]), pt global assessment of arthritis (1.4 [0.9, 2.0]), and morning stiffness (1.5 [1.0, 2.3]) vs cyclers.
Conclusion: Our findings in this sample of PsA pts in a real-world setting suggest that switching to a different MOA may lead to comparable or better outcomes vs cycling among TNFi. While further confirmatory studies are warranted, our results indicate physicians may consider non-TNFi biologics as an appropriate option when pts discontinue 1st line TNFi therapy.
To cite this abstract in AMA style:
Ogdie A, McLean R, Blachley T, Middaugh N, Mittal M, Clewell J, Ciecinski S, Mease P. The Impact of Second-Line Therapeutic on Disease Control After Discontinuation of First Line TNF Inhibitor in Patients with PsA: Analysis from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-second-line-therapeutic-on-disease-control-after-discontinuation-of-first-line-tnf-inhibitor-in-patients-with-psa-analysis-from-the-corevitas-psoriatic-arthritis-spondyloarthritis-regis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-second-line-therapeutic-on-disease-control-after-discontinuation-of-first-line-tnf-inhibitor-in-patients-with-psa-analysis-from-the-corevitas-psoriatic-arthritis-spondyloarthritis-regis/