Session Information
Date: Monday, October 27, 2025
Title: (1123–1146) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: A EULAR task force developed classification criteria (CC) for Haemochromatosis Arthropathy (HA) using a cohort of people with the C282Y homozygous mutation and arthropathy with iron overload requiring phlebotomy (1). The CC include 8 items covering age of symptom onset, clinical and X-ray features at MCP, DIP and ankle joints, and surgery of hips or ankles. A score of ≥5/11 provides 93.3% specificity and 71.4% sensitivity for classifying HA.Two additional cohorts were recruited, both without iron overload; 1. Arthropathy and any HFE gene mutation, and 2. Disease mimics (OA, CPPD). This study aimed to assess the role of iron overload and HFE gene mutations on joint disease expression by comparing the performance of the HA CC and its individual items across the 3 cohorts.
Methods: The HA cohorts included 373 patients from 12 centres across 10 countries. Group 1 (G1, n=154): C282Y homozygous with arthropathy and iron overload; Group 2 (G2, n=99): any HFE mutation with arthropathy and no iron overload; Group 3 (G3, n= 120): disease mimics (OA, CPPD).We compared features of arthropathy in G1 with G2, and G2 with G3, also with G2 subgroups: C282Y homozygous and heterozygous, H63D homozygous and heterozygous, C282Y/H63D compound heterozygous, any C282Y and compound heterozygotes, any H63D and compound heterozygotes. The total CC score, prevalence of the 8 items and proportion of patients scoring ≥5/11 to fulfil CC, was compared between groups using the Chi-square/Fisher’s exact tests or the independent sample T-test, as appropriate.
Results: Six of eight CC items were significantly more prevalent in G1 vs G2, and 5 of 8 items in G2 vs G3. The mean total score in G1 was 6.0, G2 4.3, p < 0.001 (G1 vs G2), and G3 2.7, p < 0.001 (G2 vs G3). The CC for HA were met in 71.4% of G1, 37.4% of G2 and 6.7% of G3 (p < 0.001 G1 vs G2 and G2 vs G3).Genotype-stratified analysis of G2 revealed a consistent hierarchy in prevalence of 5 of 8 of the individual CC items, total CC score and fulfilment of the CC overall. The highest prevalence was seen in people with H63D homozygous or heterozygous mutations combined, followed by any H63D and compound heterozygotes, followed by either any C282Y and compound heterozygotes or C282Y homozygous or heterozygous mutations alone (Table 1 & 2).
Conclusion: The discriminatory features of joint disease in HA, as defined by the CC, are significantly more prevalent in patients with iron overload and the C282Y homozygous HFE mutation than in patients without iron overload and any HFE genotype, supporting the major role of iron in disease expression. In patients without iron overload and with any HFE mutation, the discriminatory features of HA are significantly more prevalent than found in OA and CPPD disease mimics, supporting an iron-independent effect of HFE mutations on arthropathy. This appears to be most evident in patients with any H63D mutation, though subgroup numbers are low. Overall, these findings increase our understanding of the interplay between HFE mutations and iron status in the pathogenesis of HA, where HFE mutations alone are associated with the phenotypic features of HA, becoming much accentuated with iron loading.Reference: < !1. Kiely et al EULAR Classification Criteria for Haemochromatosis Arthropathy. ARD 2025; POS0558
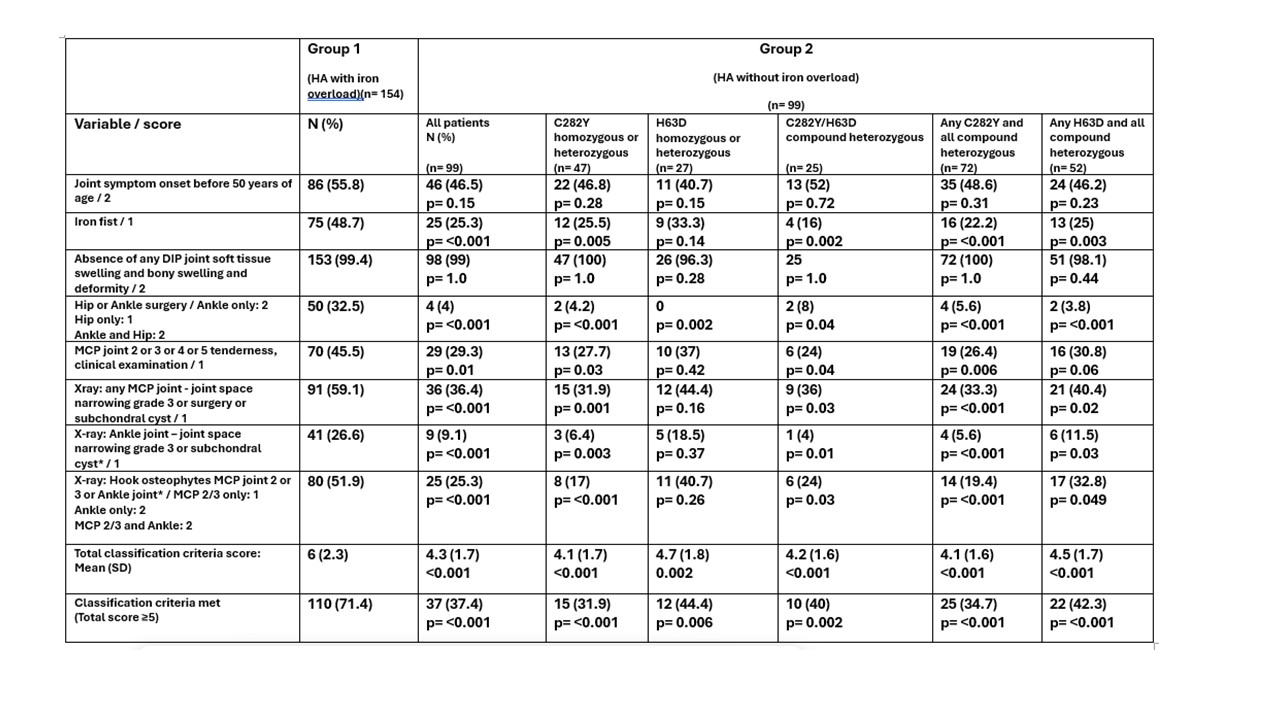 Table 1. Comparison of EULAR HA Classification Criteria component items, total score and fulfilment of the criteria in HFE C282Y homozygous patients with iron overload (Group 1) and patients without iron overload, stratified by HFE Mutation Status (Group 2).
Table 1. Comparison of EULAR HA Classification Criteria component items, total score and fulfilment of the criteria in HFE C282Y homozygous patients with iron overload (Group 1) and patients without iron overload, stratified by HFE Mutation Status (Group 2).
p values refer to comparisons with Group 1, statistical significance p < 0.05
.jpg) Table 2. Comparison of EULAR HA Classification Criteria Features in patients with HFE mutations with no iron overload (Group 2) and patients with disease mimics (OA, CPPD, Group 3). Group 2 stratified by HFE Mutation Status.
Table 2. Comparison of EULAR HA Classification Criteria Features in patients with HFE mutations with no iron overload (Group 2) and patients with disease mimics (OA, CPPD, Group 3). Group 2 stratified by HFE Mutation Status.
p values refer to comparisons with Group 3, statistical significance p < 0.05
To cite this abstract in AMA style:
Farisogullari B, Machado P, Finzel S, Carroll G, mcCarthy G, Stack J, Parisi S, Porto G, pascal r, Nagy G, Weidl M, Rosenthal A, Guggenbuhl P, Banaszkiewicz K, Butzeck B, Don H, Engelhardt S, Shearman J, Mitchell D, Barker J, Brueton V, Coathup P, Dowsett J, Duncan M, Dunleavy T, Fish I, Hoggarth A, McKinnon M, Minter J, Osborne T, Smith M, Wright C, Kiely P. The Impact of Iron Overload and HFE Genetic Mutations on Joint Disease in Haemochromatosis: Data from the Haemochromatosis Arthropathy Inception Cohorts [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-iron-overload-and-hfe-genetic-mutations-on-joint-disease-in-haemochromatosis-data-from-the-haemochromatosis-arthropathy-inception-cohorts/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-iron-overload-and-hfe-genetic-mutations-on-joint-disease-in-haemochromatosis-data-from-the-haemochromatosis-arthropathy-inception-cohorts/
