Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Ankylosing spondylitis (AS) is a chronic inflammatory disease involving the sacroiliac joints and spine and associated with pain, stiffness, and disability.1 A previous analysis of 16-wk data from the phase 3 MEASURE studies showed that secukinumab (SEC), a selective inhibitor of interleukin 17A, led to significant improvements in efficacy outcomes vs placebo, regardless of age or time since diagnosis (TSD).2 Younger patients (pts) reported better responses, possibly due to shorter TSD and higher rates of biologic inexperience. However, the relative importance of these factors was not clear.
In this hypothesis-generating analysis of pooled 52-wk data, we investigated the impact of age, TSD, TNFi exposure, and other baseline factors on responses in pts with AS treated with SEC in the MEASURE studies.
Methods: Pts with active AS from MEASURE 1 (NCT01358175), 2 (NCT01649375), 3 (NCT02008916), and 4 (NCT02159053) were included.3-8 Pts received subcutaneous (SC) SEC every 4 wk at doses of 300 mg with an intravenous (IV) loading dose (MEASURE 3 only) or 150 mg with or without an IV or SC loading dose. Pooled efficacy outcomes (ASAS20/40; ASDAS-CRP inactive disease [ID]; change from baseline [BL] in BASDAI, BASFI, and BASMI) at wk 52, were analyzed using logistic regression or ANCOVA with TSD and age at BL as continuous predictors along with key binary factors (eg, TNFi exposure). No adjustments were made for multiple comparisons.
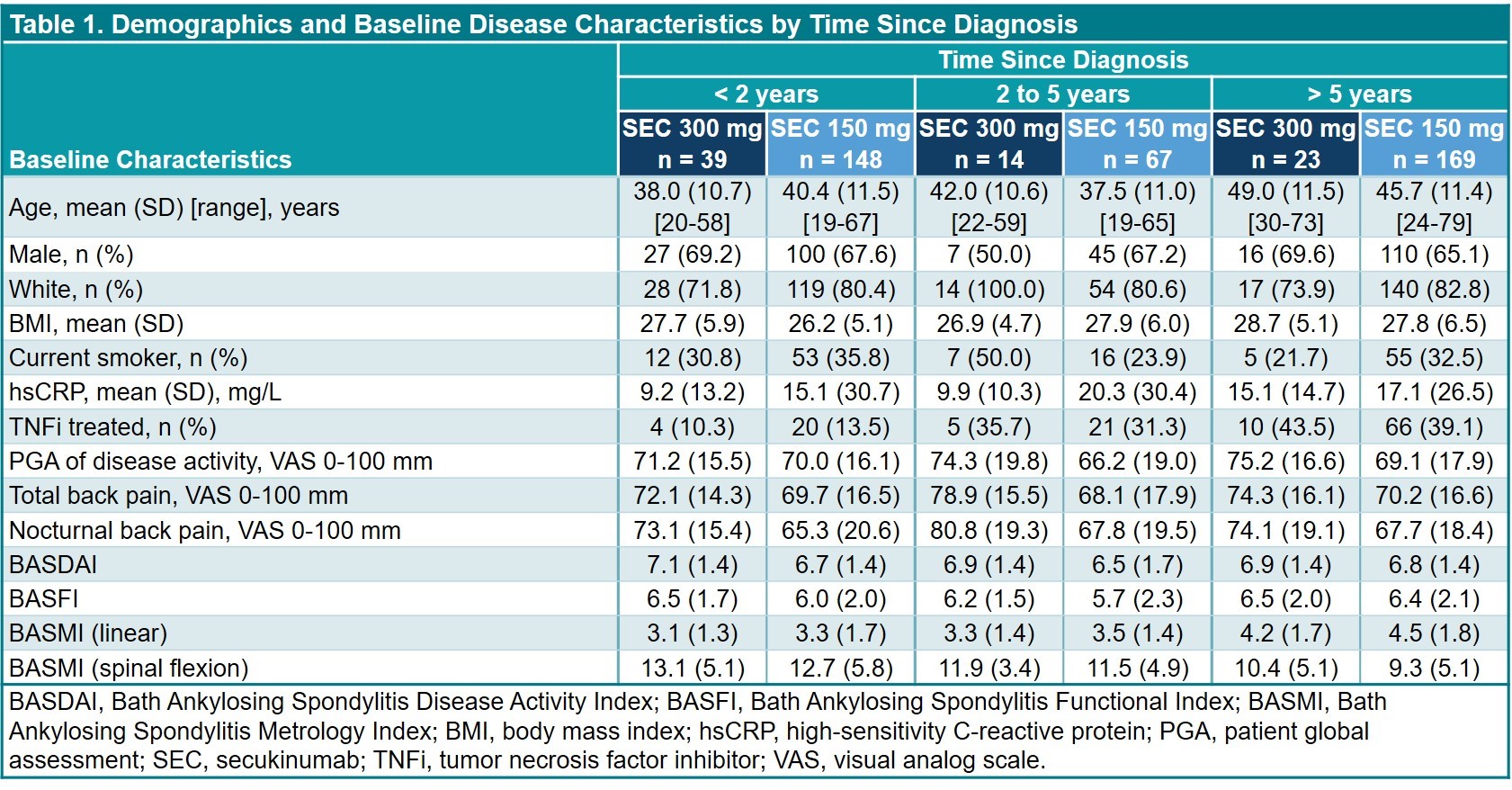
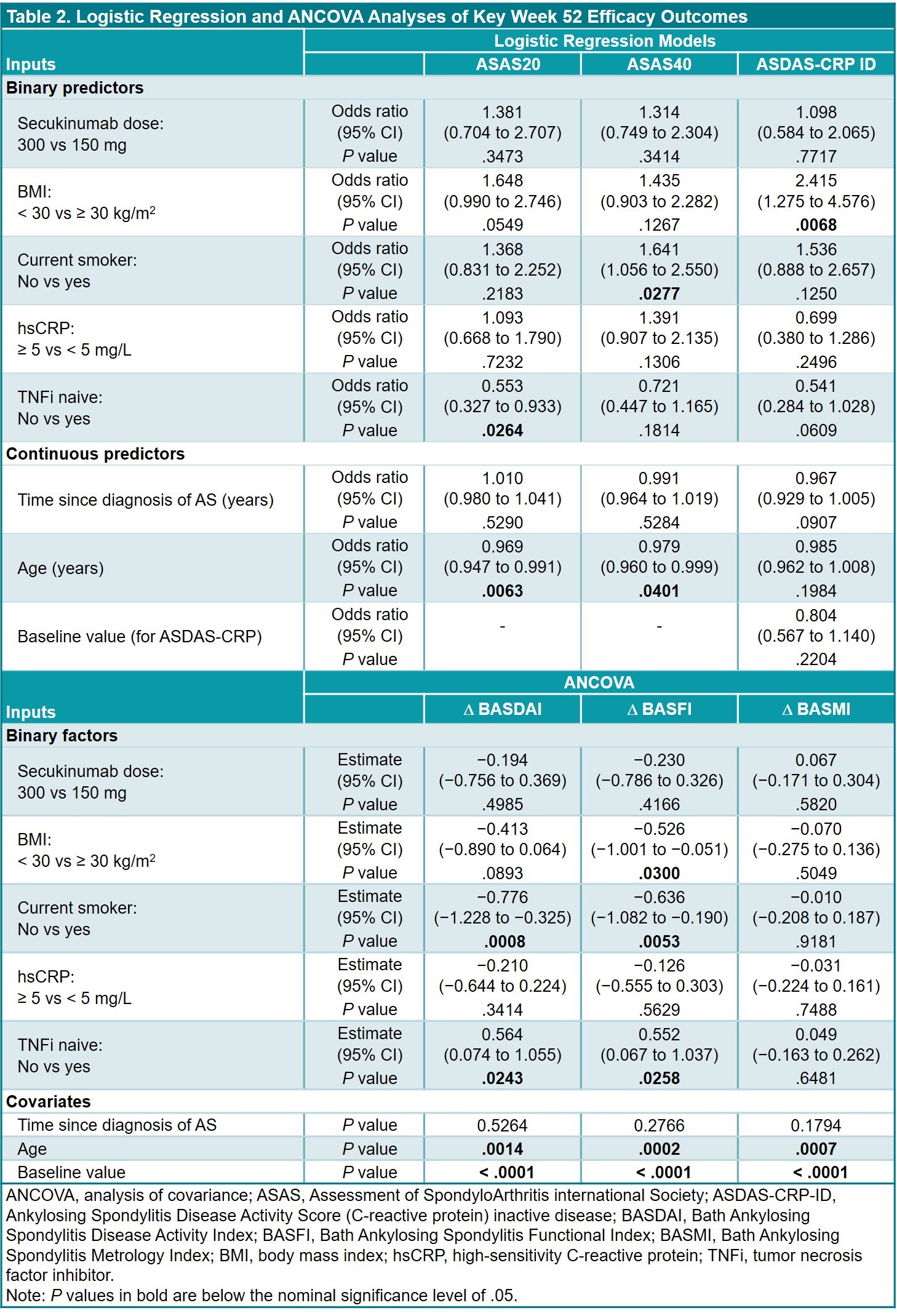
Results: This analysis included 460 pts (SEC 300 mg, n = 76; SEC 150 mg, n = 384) (Table 1); data were available at BL and wk 52 for 393 pts (85.4%). Regression analyses (Table 2) revealed that ASAS20 responses were less likely with older age and prior TNFi exposure; ASAS40 responses were more likely in nonsmokers vs smokers and less likely with older age. ASDAS-CRP ID responses were more likely to occur in pts with BMI < 30 vs ≥ 30 kg/m2. Reductions in BASDAI and BASFI were greater in nonsmokers vs smokers, and in TNFi-naive vs -treated pts (Table 2). Reductions in BASFI were also greater in pts with BMI < 30 vs ≥ 30 kg/m2. TSD was not a significant covariate. Age at BL was a significant factor for reductions in BASDAI, BASFI, and BASMI scores.
Conclusion: ASAS20/40 responses at wk 52 were more likely to occur in younger pts, and age at BL was a significant covariate for predicting reductions in disease activity measures. Better outcomes in younger pts demonstrate the importance of early treatment. It is possible that the effect of age was due to longer disease duration in older pts (which would also support the importance of early treatment) and that TSD was not predictive of outcomes due to the variable time between onset of symptoms and diagnosis; age might therefore be a better surrogate for disease duration than TSD.
- Braun J, Sieper J. Lancet. 2007;369:1379-1390.
- Deodhar A, et al. Arthritis Rheumatol. 2019;71(suppl 10):1509.
- Baeten D, et al. N Engl J Med. 2015;373:2534-2548.
- Wei JC, et al. Int J Rheum Dis. 2017;20:589-596.
- Baraliakos X, et al. Clin Exp Rheumatol. 2018;36:50-55.
- Marzo-Ortega H, et al. RMD Open. 2017;3:e000592.
- Pavelka, K, et al. Arthritis Res Ther. 2017;19:285.
- Kivitz, AJ, et al. Rheumatol Ther. 2018;5:447-462.
To cite this abstract in AMA style:
Deodhar A, Mease P, Machado P, Pournara E, Meng X, Strand V, Magrey M. The Impact of Age and Time Since Diagnosis on Response to Treatment with Secukinumab in Pooled Week 52 Data from 4 Phase 3 Studies in Patients with Ankylosing Spondylitis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-impact-of-age-and-time-since-diagnosis-on-response-to-treatment-with-secukinumab-in-pooled-week-52-data-from-4-phase-3-studies-in-patients-with-ankylosing-spondylitis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-age-and-time-since-diagnosis-on-response-to-treatment-with-secukinumab-in-pooled-week-52-data-from-4-phase-3-studies-in-patients-with-ankylosing-spondylitis/