Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Treatment response rates in lupus nephritis (LN) remain suboptimal, highlighting the need for a better understanding of LN pathogenesis to enhance treatment strategies. Single-cell transcriptomic studies are providing an unprecedented catalog of cell states in LN, yet their spatial organization is not well understood. Given that structure underlies function, our objective was to delineate the spatial organization of immune cells in LN.
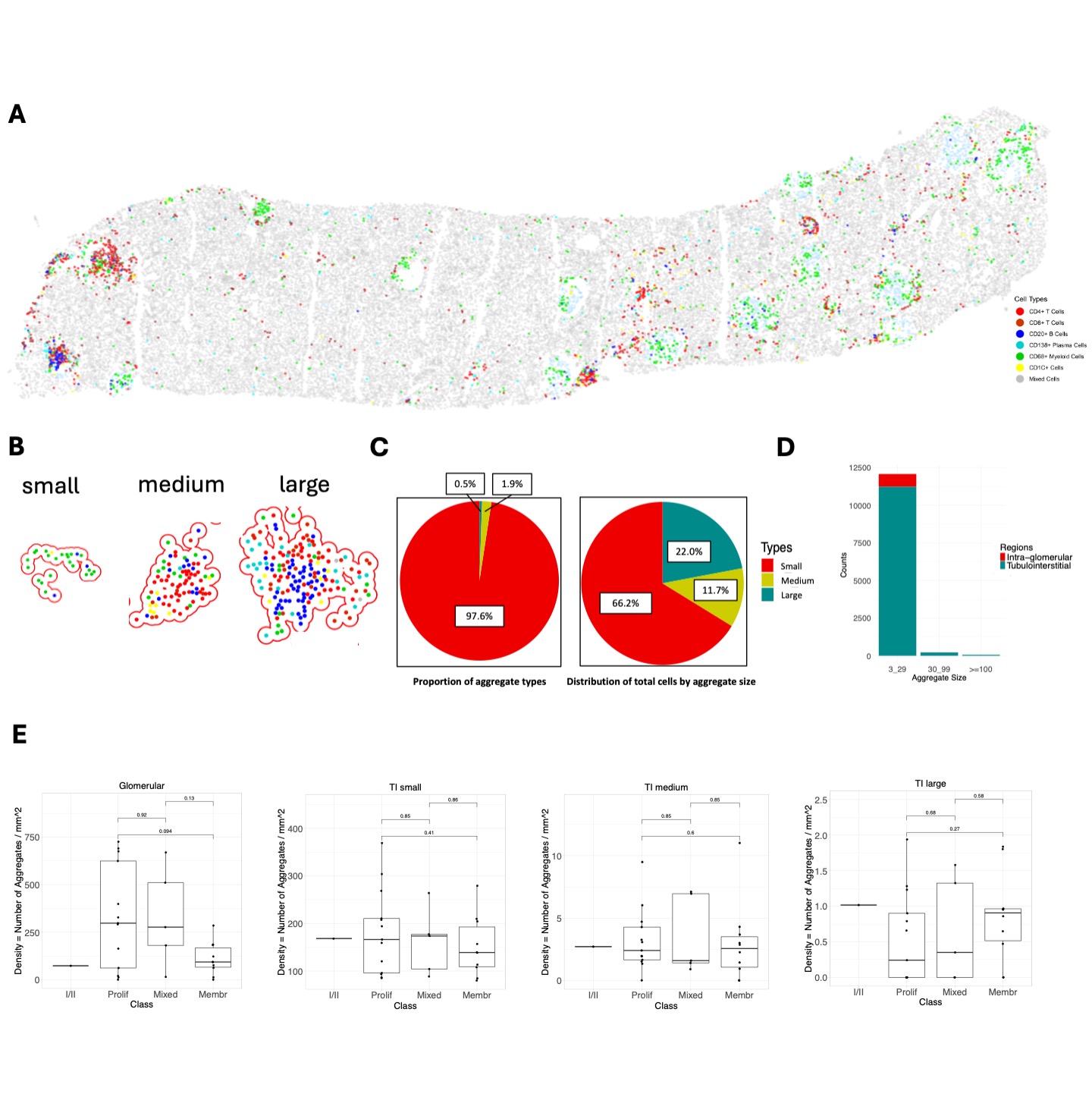
Methods: We developed a serial immunohistochemistry (sIHC) workflow (18-plex), consisting of cycles of staining, imaging, and de-staining. Image processing was carried out using HALO (Indica Labs), including AI-assisted tissue classification. Cell type annotation was performed at low resolution clustering for this preliminary analysis. Immune cell aggregates were defined using DBSCAN as a minimum of 3 cells within a radius (epsilon) of 50 μm to infer interactions between cells. Tubulointerstitial (TI) aggregate sizes were categorized into small (3-29 cells), medium (30-99 cells), and large ( >100 cells) based on the frequency distribution (Fig. 1A-B). Glomerular infiltrates were small and were not subdivided.
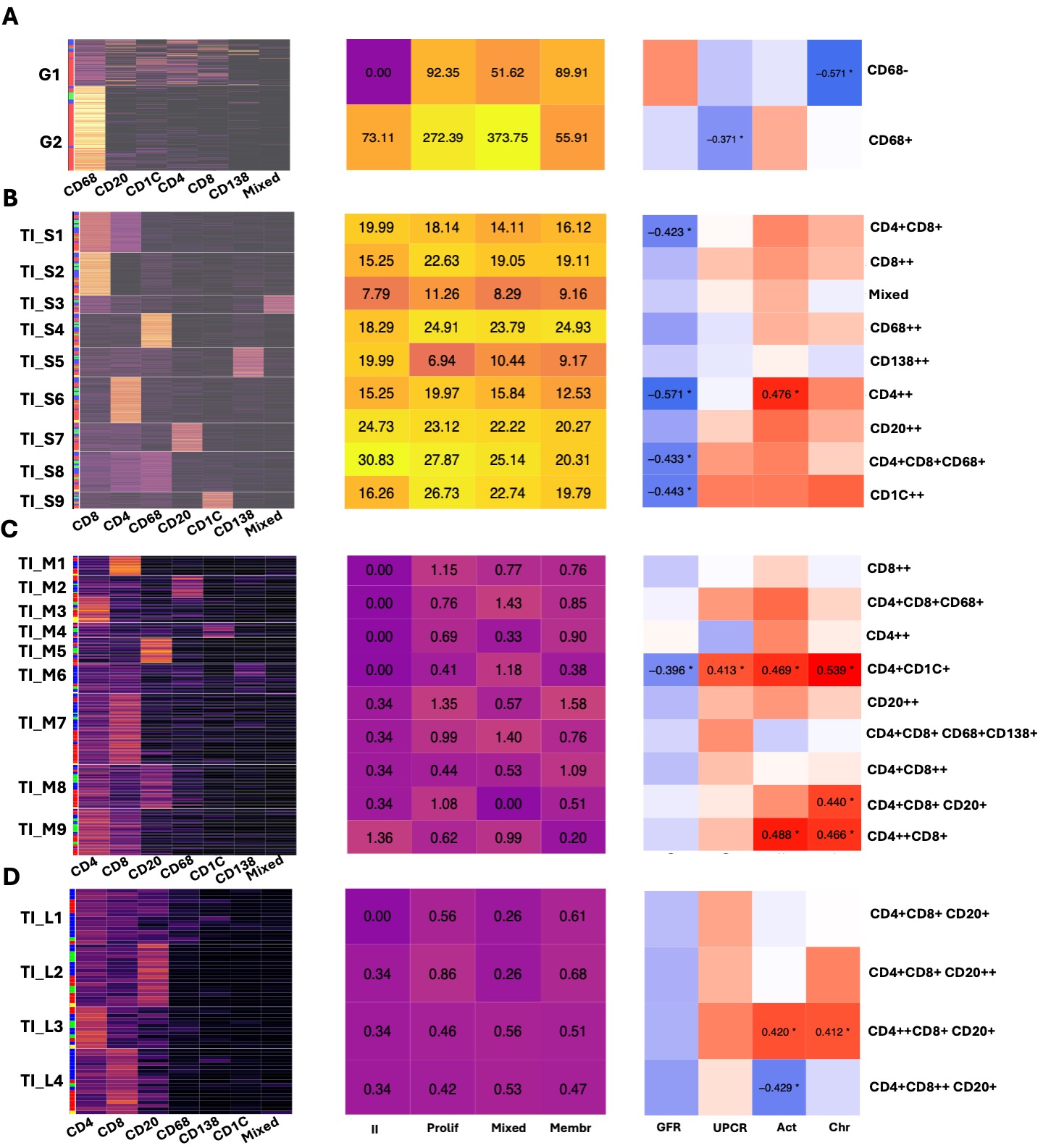
Results: In this analysis, we included 29 clinically indicated kidney biopsies classified as LN (13 pure proliferative, 10 pure membranous, 5 mixed, and 1 ISN class II) resulting in 1,913,845 cells (182,783 immune cells). We identified 12,371 cellular aggregates. Most (97%) aggregates were small (< 30 cells) (Fig. 1C-D), however, medium and large aggregates included 33.7% of immune cells. Overall, aggregate density was similar across LN classes, irrespective of size and region. Glomerular aggregates were small and primarily composed of CD68+ myeloid cells although some aggregates included T, B, dendritic and, occasionally, plasma cells (Fig. 2A). Proliferative and mixed classes had more CD68-rich aggregates, but some were also found in pure membranous. Glomerular lymphocyte-rich aggregates negatively correlated with chronicity (Fig. 2A). TI aggregate density was similar across LN classes (Fig. 1E) and negatively correlated with GFR. Significant heterogeneity in aggregate composition revealed >10 aggregate subtypes according to composition and size (Fig. 2). Small aggregates tended to be restricted to 1-2 cell types each, while medium and large aggregates included mixed proportions of CD4+ T, CD8+ T, B, dendritic, myeloid, and plasma cells likely representing germinal center-like structures (Fig. 2). Distinct TI aggregate subtypes associated with specific clinical and pathological features (Fig. 2B-C).
Conclusion: We describe the heterogeneity in glomerular and TI immune cell structures in LN, offering insights into LN pathological processes and potential cellular interactions based on proximity. Tubulointerstitial infiltrates appear similar in membranous and proliferative LN, yet specific immune structures are linked to distinct clinical and pathological features with many linked to worse GFR, regardless of class. Analysis of 90 additional biopsies is underway to validate and expand these findings.
To cite this abstract in AMA style:
Lee C, Marlin M, Yang X, Celia A, Morkotinis V, Furie R, Buyon J, Putterman C, Barnas J, Kalunian K, Izmirly P, Diamond B, Davidson A, Kamen D, Hodgin J, Accelerating Medicines Partnership RA/SLE t, James J, Petri M, Guthridge J, Rosenberg A, Fava A. The Immune Map of Lupus Nephritis: A Spatially Resolved Kidney Proteomic Approach [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-immune-map-of-lupus-nephritis-a-spatially-resolved-kidney-proteomic-approach/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-immune-map-of-lupus-nephritis-a-spatially-resolved-kidney-proteomic-approach/