Session Information
Date: Saturday, November 16, 2024
Title: SLE – Animal Models Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: TREX1 gene encodes the three prime repair exonuclease 1 enzyme that degrades DNA. TREX1 plays a key role in genomic DNA degradation, cell death processes, and gap-filling during DNA repair. The most significant role of TREX1 is to maintain host innate immune tolerance to cytosolic self-DNA by degrading a range of substrates to prevent initiation of autoimmunity via regulation of cyclic GMP-AMP synthase(cGAS) – Stimulator of interferon Genes (STING) immune signaling. TREX1 mutations have been associated with autoimmune disorders, including Aicardi-Goutières Syndrome, familial chilblains lupus. However, it is unclear whether mutations in TREX1 play a causal role in the disease progression, and whether manipulation of TREX1 has a beneficial effect in the treatment of autoimmune diseases. The role of TREX-2 in DNA repair has been studied in C. elegans but not TREX-1. We proposed to study the mechanism of disease caused by TREX-1 mutations, linked to Lupus in C. elegans.
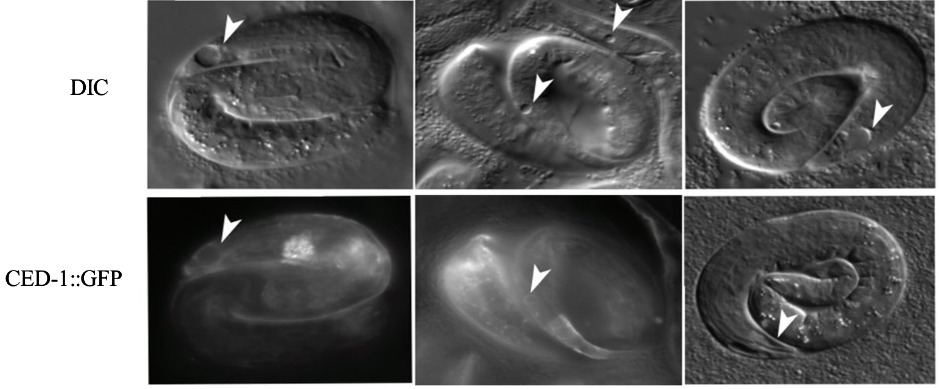
Methods: We found evidence that genomic sequence W02F12.4 in C. elegans is the ortholog of TREX1. We used CRISPR to recreate point mutations in the C. elegans TREX-1 gene and characterize their phenotypes. We also used RNAi (RNA interference) to deplete the TREX1 mRNA, by feeding C. elegans worms bacteria carrying double stranded RNA of the trex-1 gene. C. elegans has a well-studied receptor for apoptotic corpse clearance called CED-1. Based on sequence alignment, we proposed SCARF1 is an ortholog of CED-1. We used CED-1: GFP to detect signal around dying cells via live imaging.
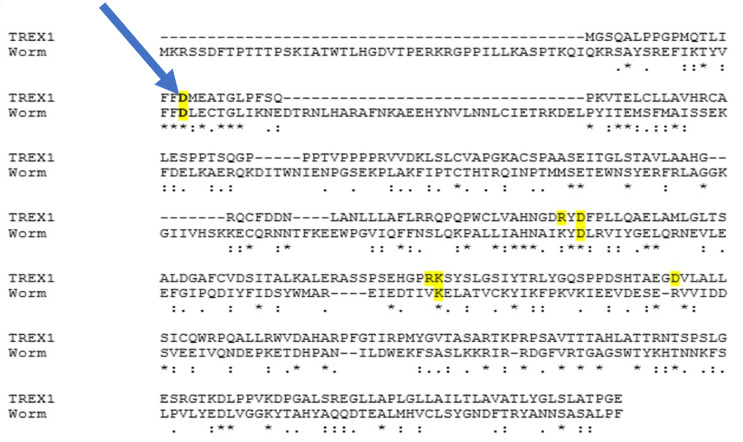
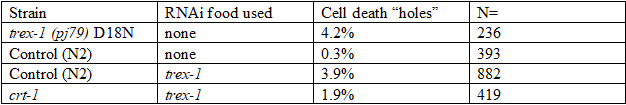
Results: We used two methods to interfere with TREX1 function in C. elegans: RNAi depletion and CRISPR. 1. RNAi depletion: Three days after feeding C. elegans worms bacteria carrying double stranded RNA of the trex-1 gene, the progeny at the late embryonic “pretzel” stage had holes in the head and tail region. This “holes” phenotype looks like necrotic cell death. The dead cells are recognized by the CED-1/SCARF cell death receptors (Fig. 1). 2. CRISPR: We found that D18, K175, D 130 amino acids that are mutated in TREX1 in connection with Lupus were conserved in C. elegans via sequence alignment (Fig.2). We first created the D18N mutation in TREX-1. Similar to the RNAi results, we saw that some of the developing worms had holes, that appeared necrotic. To test if the holes are necrotic, we tested if they depend on calreticulin, a protein that helps export Calcium from the endoplasmic reticulum during necrotic cell death. A mutation in calreticulin, crt-1, can block necrotic deaths. Feeding trex-1 RNAi to crt-1 worms showed a decrease in holes but did not completely block them (Table1).
Conclusion: Using C. elegans, a small model organism with existing tools for phenotypic analysis, live imaging of dying cells, and known genetics pathways that regulate corpse engulfment, we discovered that TREX-1 loss results in cell deaths that look necrotic. If further evidence supports that loss of TREX-1 causes necrotic death, we can investigate which pathways leading to necrotic death may be affected by the loss of TREX-1. Knowing the exact pathway could identify molecular regulators of TREX1. It is possible that TREX1 mutations contribute to cell deaths that result in inflammation that contribute to autoimmunity.
To cite this abstract in AMA style:
Kyi H, Barreto P, Pulijala Y, Venkatachalam T, Soto M. The Human Lupus TREX1 D18N Mutation Engineered in C. Elegans Leads to Cell Death. What Can We Learn About Lupus Using C. Elegans? [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-human-lupus-trex1-d18n-mutation-engineered-in-c-elegans-leads-to-cell-death-what-can-we-learn-about-lupus-using-c-elegans/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-human-lupus-trex1-d18n-mutation-engineered-in-c-elegans-leads-to-cell-death-what-can-we-learn-about-lupus-using-c-elegans/