Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The high dose trivalent inactivated influenza vaccine (HD-TIV) contains four times the antigen dose per strain of the standard-dose inactivated influenza vaccines. HD-TIV is licensed only for persons aged 65 years and older based on immunogenicity and efficacy data. In seropositive RA, HD-TIV substantially improves the immune response to vaccination compared to a quadrivalent standard dose influenza vaccine (SD-QIV). It is unknown whether among RA patients, the increased immunogenicity of the HD-TIV is restricted to a specific age subgroup.
Methods: Between 2016 and 2018, we conducted a treatment-stratified, randomized, modified double-blind, active-controlled trial in adult seropositive RA patients (rheumatoid factor and/or anti-cyclic citrullinated peptide antibodies) to assess antibody responses to either SD-QIV (15 μg of hemagglutinin per strain) or HD-TIV (60 μg of HA per strain) (NCT02936180). Seroconversion (SC) rates were assessed using pre- (Day 0 – D0) and post-vaccine (D28) titers determined by serum hemagglutination inhibition (HI) and microneutralization (MN) assays. In both cases, SC was defined as at least a four-fold HI or MN antibody titer increase from D0.The effect of age (adults versus elderly, cutoff age 65+ years) on SC rates were evaluated using logistic regression analysis.
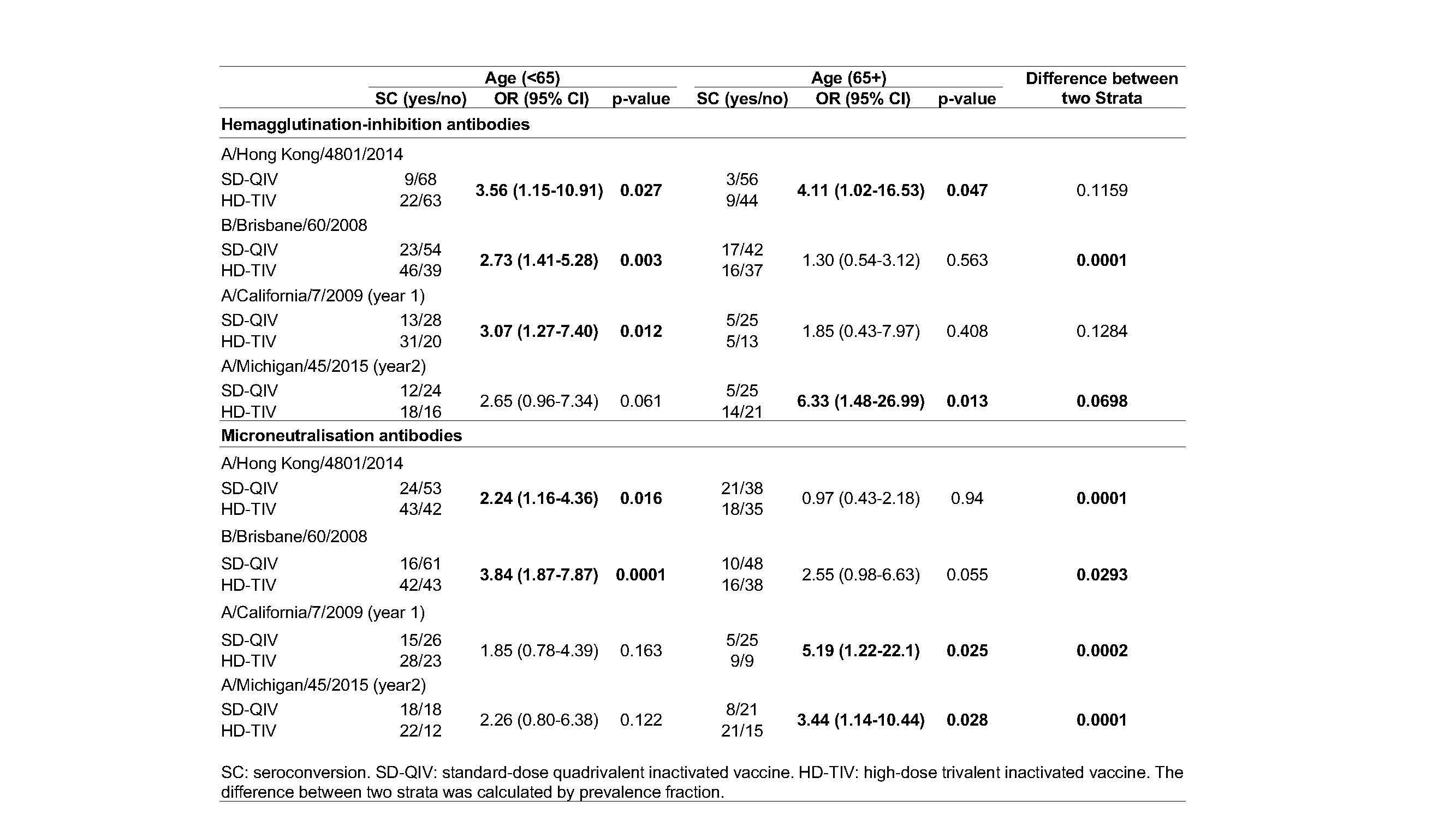
Results: A total of 279 seropositive RA patients were enrolled. 140 (50.2%) received SD-QIV and 139 (49.8%) received HD-TIV. The mean age (±SD) was 61.0±12.9 and 80% were female. Results of seroconversion rates based on hemagglutination and microneutralization assays, per vaccine strain and according to RA age groups are presented in Table 1.
Conclusion:
The benefit of the HD-TIV regarding immune protection is not restricted to elderly seropositive RA patients. The HD-TIV substantially improves immune responses to all influenza strains in adults living with RA.
To cite this abstract in AMA style:
Useche M, Agnihotram R, Bernatsky S, Ward B, Colmegna I. The High Dose Influenza Vaccine Increases Immune Protection in Both Adults and Elderly Seropositive RA Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-high-dose-influenza-vaccine-increases-immune-protection-in-both-adults-and-elderly-seropositive-ra-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-high-dose-influenza-vaccine-increases-immune-protection-in-both-adults-and-elderly-seropositive-ra-patients/