Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: While oral CS (OCS) are the mainstay of SLE therapy, prolonged use is associated with organ damage and early mortality.1,2 Minimizing OCS use is among SLE treatment goals.1 The beneficial effect of belimumab (BEL) on OCS reduction was shown in two pooled analyses of five randomized clinical trials,3 and six retrospective observational real-world studies.4
This study used real-world data from a large rheumatology provider network to compare OCS use in patients (pts) with SLE in the US before and after BEL initiation.
Methods: This retrospective cohort study (GSK Study 214140) utilized data from the Patient-Important Outcomes Data Repository (PIONEER)-Rheumatology database.5 Eligible adults with SLE initiated BEL between Jan 1, 2012, and Jun 30, 2021, and had available data for ≥180 days before and ≥360 days post BEL initiation. The index was the date of BEL initiation. Primary objectives: proportion of pts receiving any OCS; mean total OCS dose per pt; mean total number of OCS days supplied per pt; mean daily OCS dose for days supplied per pt (in pts with ≥1 OCS prescription); proportion of pts with daily OCS dose of ≤5 mg and ≤7.5 mg for days supplied. A secondary objective was to describe pt clinical characteristics during follow-up. Changes in OCS use were assessed between Period (P) 1 (180 days pre-index [−180 to –1 days]), P2 (first 6 months post index [0 to 180 days]), and P3 (second 6 months post index [180 to 360 days]) in ptswith OCS use in P1 who were adherent to BEL at each assessed period.
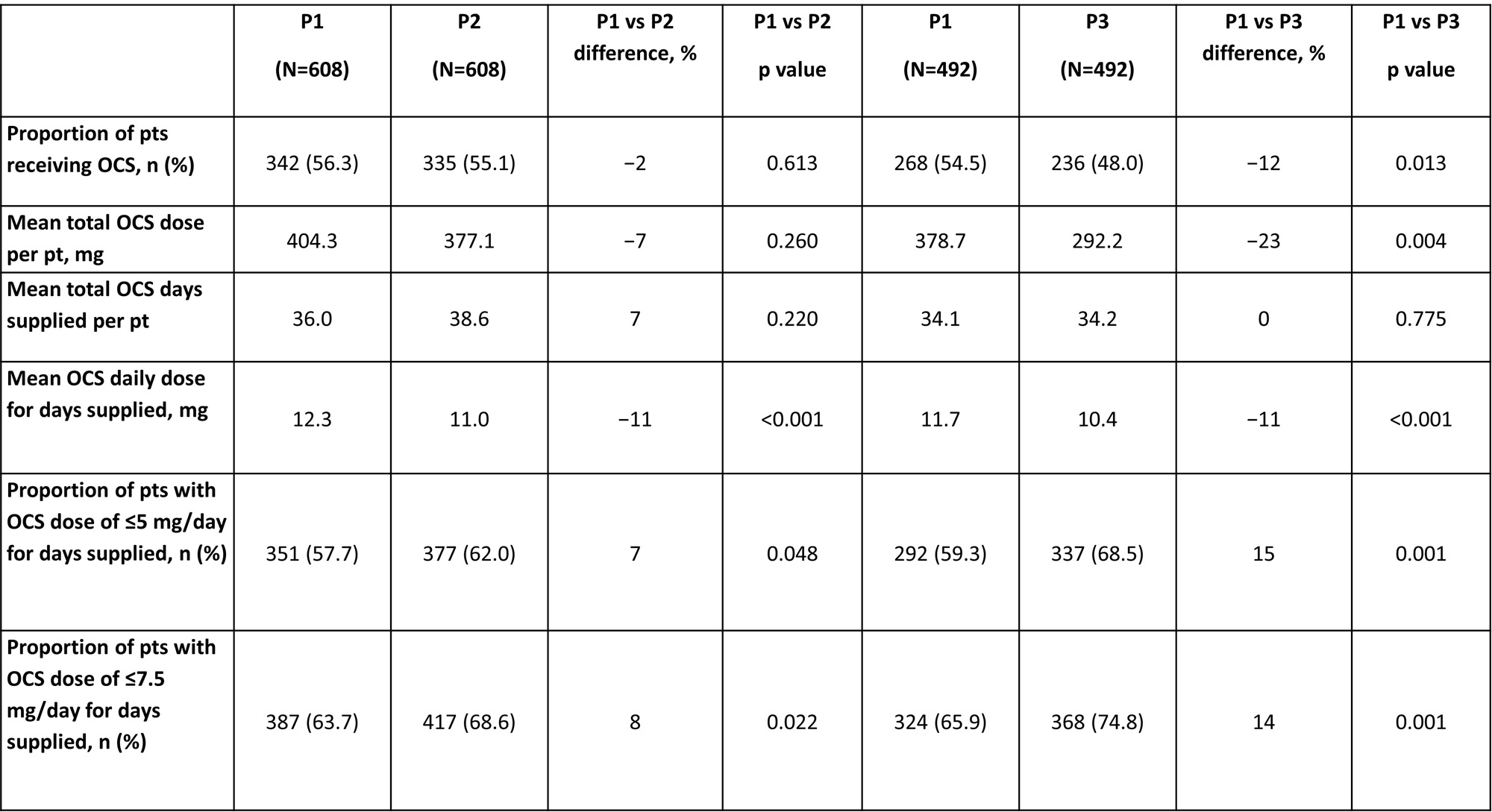
Results: Of the 14,439 pts with SLE in the database, 608 received BEL for >180 days (full analysis set, FAS; 92.8% female, 70.4% with moderate SLE) and were included; 492 pts adhered to 360 days of BEL. In P1, 56.3% of FAS pts and 54.5% of pts who adhered to 360 days of BEL were receiving OCS.
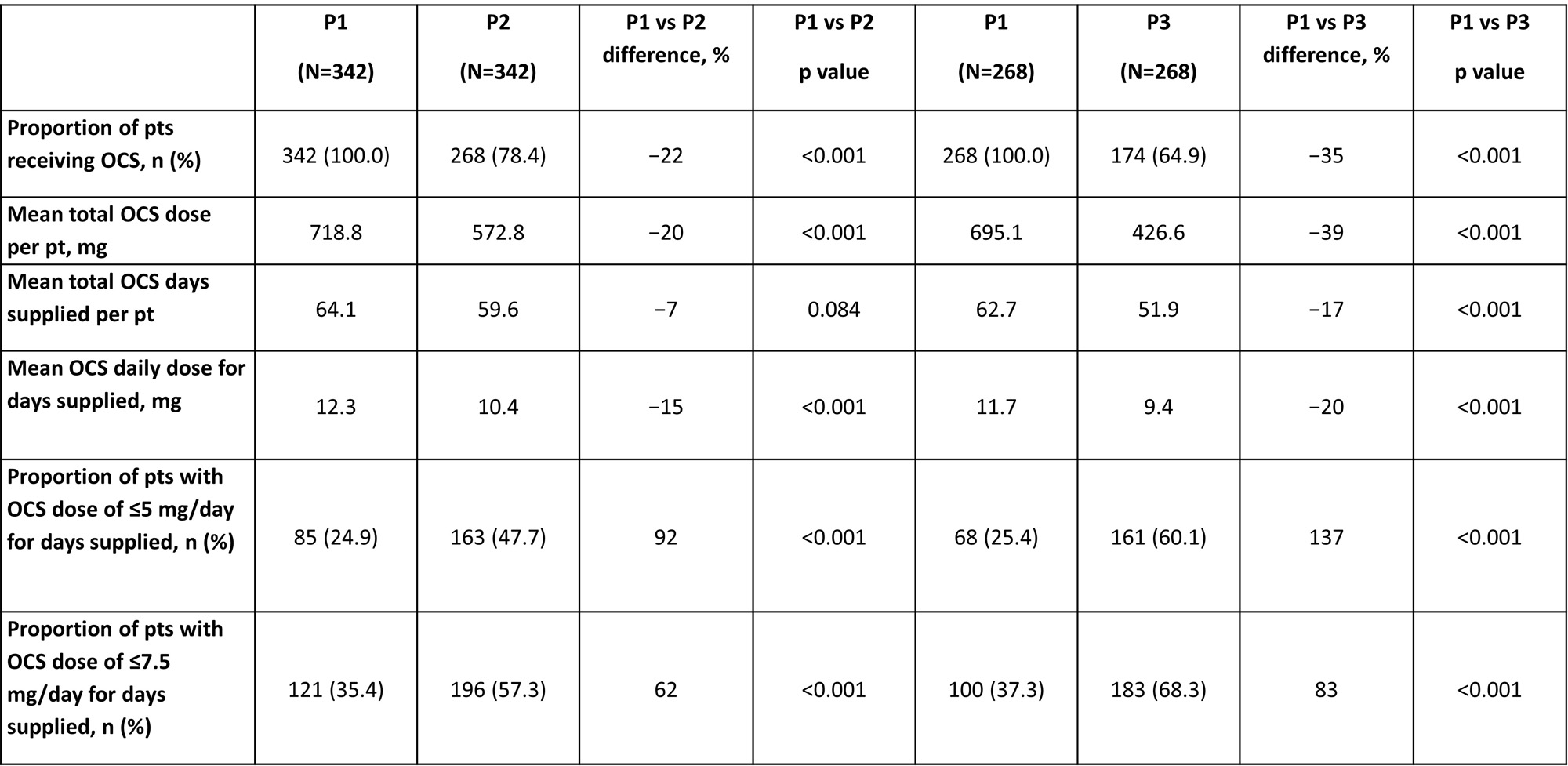
Improvements in OCS use were more pronounced in pts with OCS use in P1 (Table 1), compared with the FAS, which included pts with no OCS use in P1 (Table 2).
Among pts receiving OCS in P1, significantly fewer received OCS in P2 and P3 versus P1 (Table 1). Significant reductions from P1 were observed in P2 and P3 for mean total OCS dose per pt, mean OCS daily dose for days supplied, and mean total OCS days supplied per pt in P3 only; significantly more pts received daily OCS dose of ≤5 mg and ≤7.5 mg in P2 and P3 versus P1 (Table 1).
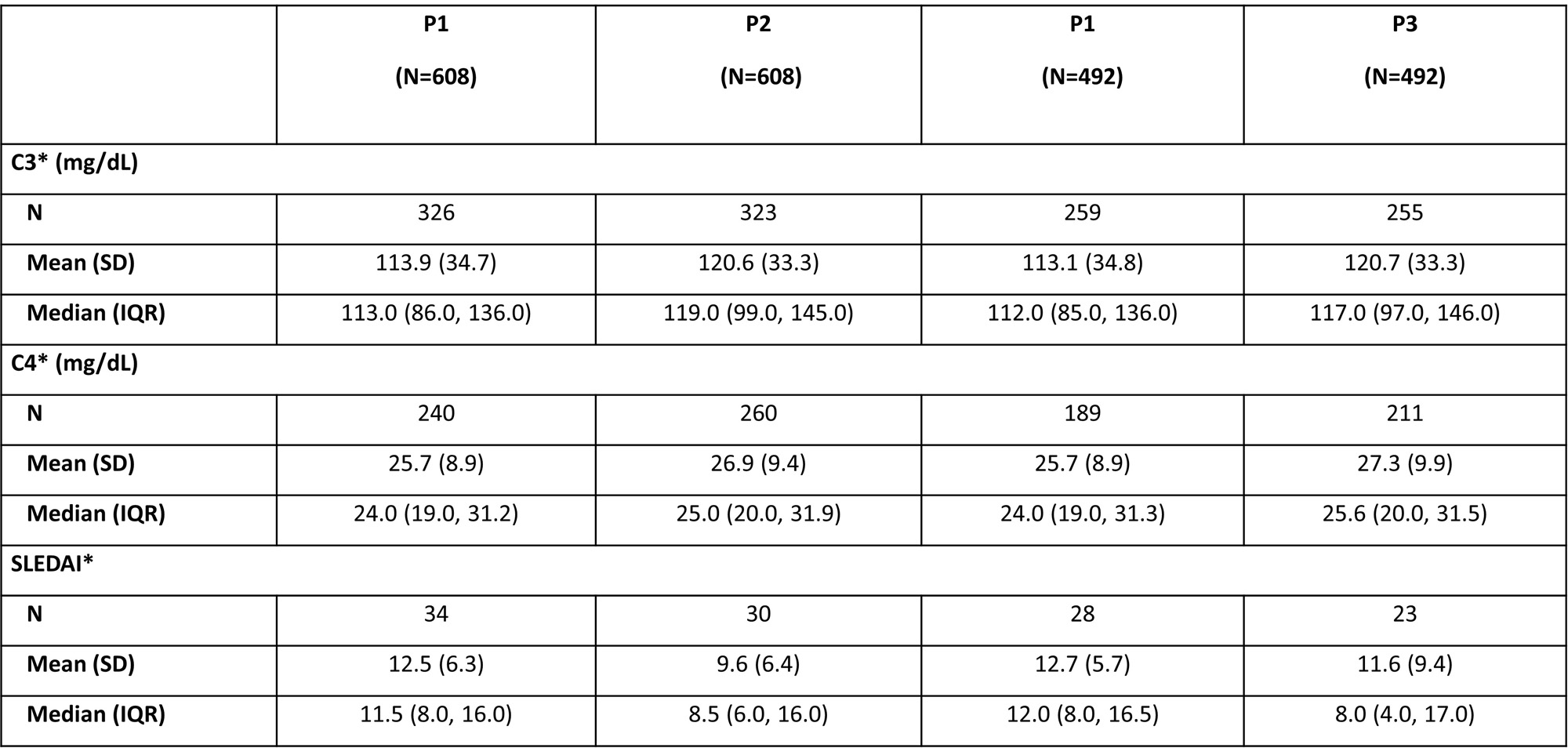
Slight increases from P1 in C3 and C4 levels were observed in P2 and P3; mean SLEDAI scores (for pts with available data for the specified period) decreased from 12.5 in P1 to 9.6 in P2 in FAS pts and from 12.7 in P1 to 11.6 in P3 in pts who adhered to 360 days of BEL (Table 3).
Conclusion: This analysis of data from a large rheumatology database showed OCS dose and use reductions with BEL in pts receiving OCS before BEL initiation, providing more real-world evidence for the steroid-sparing effect of BEL in pts with SLE and baseline OCS use. The beneficial effect was more pronounced with longer duration of BEL treatment.
Funding: GSK
References
1 Fanouriakis A et al. Ann Rheum Dis 2019;78:736–45
2 Sheane BJ et al. Arthritis Care Res 2017;69:252–6
3 Costenbader K et al. Arthritis Rheumatol 2021;73 (suppl 9)
4 Collins CE et al. Rheumatol Ther 2020;7:946–65
5 Helfgott S et al. Arthritis Rheumatol 2022;74 (suppl 9)
Note: Percentages for differences are shown after rounding to the nearest whole number. Distributions of differences between periods were assessed by Kolmogorov–Smirnov and Shapiro–Wilk tests and none of the measures were found to be normally distributed. Wilcoxon Signed Ranks test was used to generate p values. OCS dose=prednisone equivalent.
Note: Percentages for differences are shown after rounding to the nearest whole number. Distributions of differences between periods were assessed by Kolmogorov–Smirnov and Shapiro–Wilk tests and none of the measures were found to be normally distributed. Wilcoxon Signed Ranks test was used to generate p values. OCS dose=prednisone equivalent.
*Among pts with data available for the given period; data may not be for the same pts assessed in each period.
IQR, interquartile range; SD, standard deviation.
To cite this abstract in AMA style:
Worley K, Milligan S, Rubin B. The Effect of Belimumab on Steroid Use in Patients with SLE: Results from a Retrospective Observational Study of Real-World Data from a US Rheumatology Provider Network [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-effect-of-belimumab-on-steroid-use-in-patients-with-sle-results-from-a-retrospective-observational-study-of-real-world-data-from-a-us-rheumatology-provider-network/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-belimumab-on-steroid-use-in-patients-with-sle-results-from-a-retrospective-observational-study-of-real-world-data-from-a-us-rheumatology-provider-network/