Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
No objective measure is presently available to assess digital ulcer (DU) in SSc patients apart from “healed/non healed” and experience-based clinical judgment. The aim of the current study is to propose a composite DU clinical assessment score (DUCAS) and to lend it, test its face validity by correlating it with commonly used disease related patient-reported outcomes (PROs) and physician evaluation.
Methods:
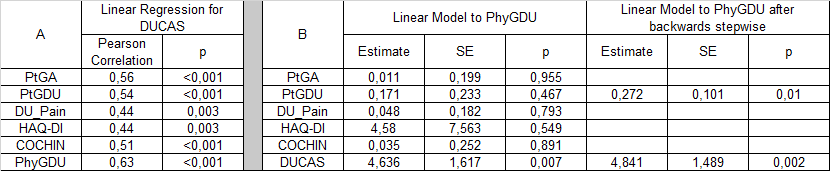
SSc patients presenting at least one DU and attending the Rheumatology Wound Care Clinic of the Florence University Hospital or the London Royal Free Hospital were enrolled. Patients were assessed with HAQ-DI, Cochin scale, Visual analogic scale (VAS) for DU-related pain (DU_pain, 0-100 mm), patient VAS for global DU status (ptGDU, 0-100mm) and patient global assessment (PtGA, 0-100 mm) as PROs and physician VAS for DU status (phyGDU, 0-100mm). The DUCAS included 7 DU related variables selected by a committee of 8 SSc DU experts – they are outlined in table 1. Each variable was weighted on a clinical basis and the DUCAS score was the sum of the values for the 7 variables (max=19,5). Spearman’s correlation tests were calculated for to examine face validity. A linear regression model with forward and backward stepwise analysis was used to determine the relationship of individual variables with the primary clinical parameter, phyGDU.
Results:
44 SSc patients (9 males, mean age 54,3±15,6 years, mean disease duration 9,9±5,8 years) were enrolled in the study. Mean phyGDU was 44,3±23mm, mean ptGDU was 54±30mm (Wilcoxon p=0.022, phyGDU VAS vs ptGDU) and mean DUCAS score was 4,2±2. Overall DUCAS showed significant positive correlations with all PROs, but when all the individual clinician and patient’s variables were modelled, only the overall DUCAS significantly predicted PhyGDU; after backwards stepwise analysis overall DUCAS and ptGDU best predicted PhyGDU, with an adjusted R²=0,437 and AIC=380,3 (Table 2).
Conclusion:
DUCAS is a newly proposed clinical score for SSc related DU which has face validity and which may reflect DU statusas judged by SSc experts. Further validation of this score will be undertaken.
To cite this abstract in AMA style:
Bruni C, Ngcozana T, Braschi F, Piemonte G, Benelli L, Guiducci S, Bellando-Randone S, Grotts J, Denton C, Furst DE, Matucci-Cerinic M. The Ducas: Proposal for a Digital Ulcer Assessment Score in Scleroderma [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/the-ducas-proposal-for-a-digital-ulcer-assessment-score-in-scleroderma/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-ducas-proposal-for-a-digital-ulcer-assessment-score-in-scleroderma/