Session Information
Date: Monday, November 18, 2024
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with rheumatoid arthritis (RA) who fail conventional synthetic treatment with disease modifying anti-rheumatic drugs (csDMARDs) are eligible for biological DMARDs (bDMARDs) or targeted synthetic DMARDs (tsDMARDs). Many patients experience a lack of response or intolerance to their first advanced therapy (AT), requiring a change in medication. Subsequent treatment choice is important for achieving successful long-term disease control. The current study aimed to describe the pattern of sequential AT use in RA patients in a multicentre observational cohort and to evaluate the survival rate and effectiveness of each line of therapy.
Methods: Adult RA patients participating in the Ontario Best Practice research Initiative (OBRI) and initiating their first AT (line 1) between Jun. 1, 2008 and Jan. 1, 2023 were included. Drug retention was defined as the time from initiation to discontinuation of therapy (due to any reason). We evaluated effectiveness using Clinical Disease Activity Index (CDAI) change, the proportion of patients reaching the minimally clinically important difference (MCID), CDAI low disease activity (LDA), and remission at 6 months. Time to event analysis was used for treatment discontinuation and general linear mix model for effectiveness. An exploratory analysis compared outcomes in patients who started their first AT before and after 2010, the year treat to target guidelines were published. We also compared drug survival of the first AT in three therapeutic groups (TNFi, non-TNFi, tsDMARDs).
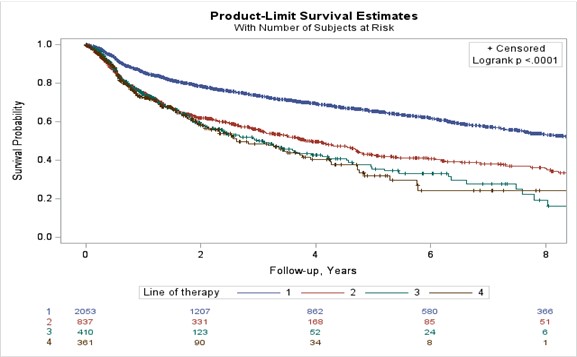
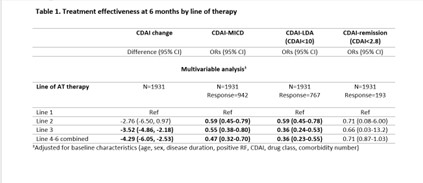
Results: A total of 2449 patients were included (line 1=1117, line 2=679, line 3=339, and lines 4 to 7=314). TNFi was predominantly used as first-line AT, with Etanercept and Adalimumab being the 1st and 2nd most common choices. Subsequent AT lines exhibited lower TNFi usage. Risk of discontinuation increased in later lines, persisting after adjustments for confounders (Figure 1). Pre-2010 and post-2010 cohorts displayed significant differences in first-line AT retention, suggesting quicker switches post-2010 (Median survival 12.2 vs 7.6 years). JAK inhibitors (JAKi) as first-line AT had lower drug survival compared to bDMARDs. Efficacy outcomes favoured first-line AT, with lower CDAI change and attainment of clinical targets in subsequent lines. (Table 1).
Conclusion: In this real-world study, we found that TNFi remains the most common first AT, however there has been a downward trend in using TNFi. The first AT has a longer survival and better efficacy when compared to subsequent lines. Clinicians tend to switch the first line therapy earlier since 2010, likely due to a shift towards a treat to target approach and more available therapeutic options.
To cite this abstract in AMA style:
Movahedi M, Cesta A, Li X, Kuriya B, Aydin S, Bombardier C, Seyed-Akhavan P. The Discontinuation and Effectiveness of Sequential Advanced Therapy in Rheumatoid Arthritis, a Real-World Data [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-discontinuation-and-effectiveness-of-sequential-advanced-therapy-in-rheumatoid-arthritis-a-real-world-data/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-discontinuation-and-effectiveness-of-sequential-advanced-therapy-in-rheumatoid-arthritis-a-real-world-data/