Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Weight-loss has been shown to alleviate knee osteoarthritis (OA) symptoms in persons with OA and obesity. Utilization of GLP1RA medications has increased rapidly. These agents result in average weight-loss of 10-25%. We sought to determine the cost-effectiveness of adding a GLP1RA (semaglutide or tirzepatide) to usual OA care (UC) for patients with obesity and symptomatic knee OA.
Methods: We used the Osteoarthritis Policy (OAPol) Model, a widely published and validated microsimulation of knee OA, to estimate the long-term clinical benefits and costs of GLP1RA treatments compared to three other weight-loss strategies and UC alone: 1) UC (NSAIDs, corticosteroid injections, opioids and total knee replacement); 2) UC + diet and exercise (D+E); 3) UC + 2.4 mg weekly semaglutide; 4) UC + 15 mg weekly tirzepatide; 5) UC + laparoscopic sleeve gastrectomy (LSG); 6) UC + Roux-en-Y gastric bypass (RYGB).We derived weight reduction associated with semaglutide and tirzepatide from the STEP-1 and SURMOUNT-4 trials, respectively. We modeled a 20% reduction in major cardiovascular events based on the SELECT trial. We used a 12% GLP1RA annual discontinuation rate derived from observational studies. In the base case analysis, we assumed a maximum GLP1RA use of 5 years. We used published literature to determine the pain reduction associated with weight-loss and the BMI reduction from other strategies. The annual costs of GLP1RA regimens include the wholesale acquisition cost of the medication itself plus monthly lifestyle coaching ($17,000 for semaglutide; $13,500 for tirzepatide). We initiated a cohort with a mean age of 55 years, WOMAC pain (0-100, 100 worst) of 38, mean BMI of 38 kg/m2 and 50% KL grade 2/50% KL grade 3. We calculated incremental cost-effectiveness ratios (ICERs) as the ratio of the difference in lifetime costs (2024 USD) to the difference in quality-adjusted life years (QALYs) between two adjacent, competing strategies. We conducted the analysis from a modified societal perspective and discounted QALYs and costs at 3% annually. We performed sensitivity analyses to examine the robustness of our findings given the uncertainty in input parameters.
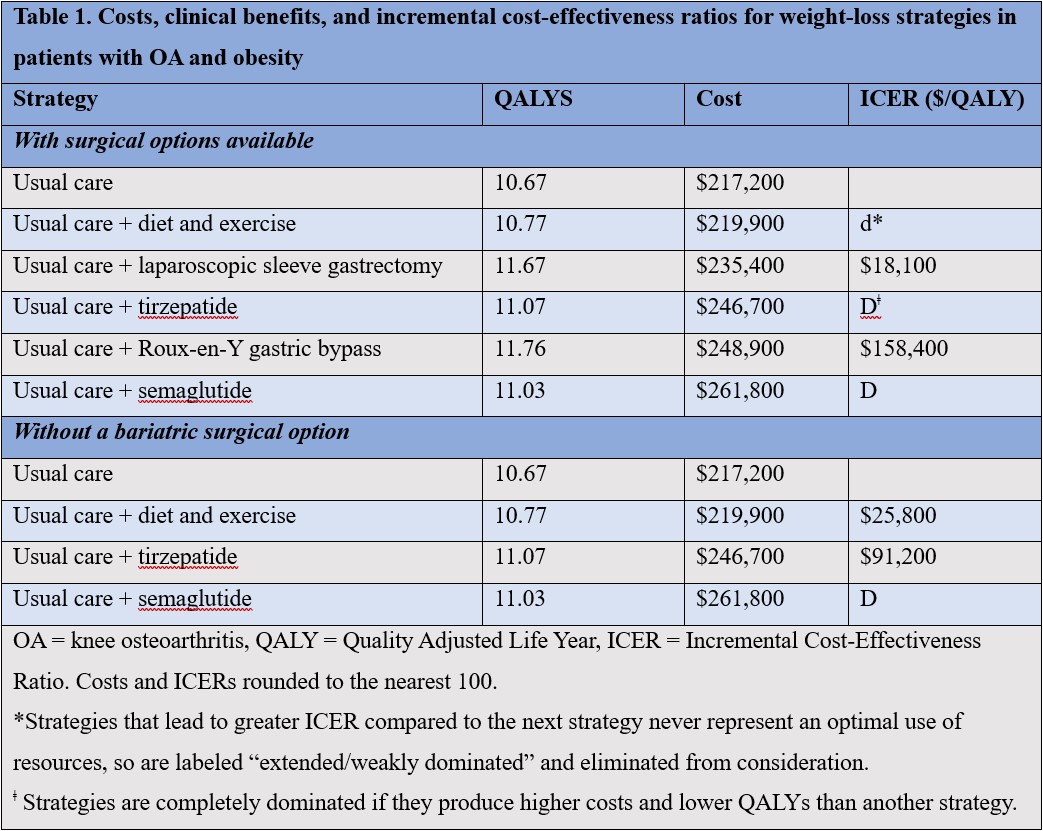
Results: GLP1RA-based strategies were dominated (increased costs and decreased QALYs) by LSG and RYGB (Table 1). The ICERs for LSG and RYGB were $18,100/QALY and $158,400, respectively. In the absence of surgical options, D+E had an ICER of $25,800/QALY, and tirzepatide’s ICER was $91,200. Medication cost, years on treatment, and starting BMI had the greatest impact on tirzepatide’s ICER: when the price of tirzepatide was lowered to its federal supply schedule cost ($9,840), its ICER fell to $56,800. D+E was cost-effective in 87% of scenarios if willingness to pay (WTP) was $50,000/QALY; tirzepatide was cost-effective in 53% if WTP was $100,000.
Conclusion: In the absence of surgical options, tirzepatide could be cost-effective if WTP < $100,000/QALY. Semaglutide was dominated by tirzepatide due to its higher costs and lower weight-loss efficacy. For WTP < $50,000, D+E could offer good value. LSG could offer high value in clinical OA management for patients with OA and obesity who are willing to undergo bariatric surgery.
To cite this abstract in AMA style:
Betensky D, Katz J, Yang C, Hunter D, Collins J, Feldman C, Smith K, Messier S, Kim J, Selzer F, Losina E. The Cost-effectiveness of Glucagon-Like Peptide-1 Receptor Agonists (GLP1RAs) for Patients with Knee Osteoarthritis and Obesity [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-cost-effectiveness-of-glucagon-like-peptide-1-receptor-agonists-glp1ras-for-patients-with-knee-osteoarthritis-and-obesity/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-cost-effectiveness-of-glucagon-like-peptide-1-receptor-agonists-glp1ras-for-patients-with-knee-osteoarthritis-and-obesity/