Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
The HLA-DRB1 shared epitope (SE) is associated with joint destruction in ACPA+ patients (pts) with RA.1 In the Early AMPLE trial, among ACPA+ pts with early RA, efficacy responses with abatacept (ABA) but not adalimumab were numerically higher in pts who were SE+ vs SE–.2 This real-world analysis compared the mean change (∆) from baseline to the 6-mth follow-up visit in CDAI score among ACPA+/SE+ pts treated with ABA or TNF inhibitors (TNFi).
Methods:
Using data from CERTAIN (Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory CoNditions; NCT01625650)3, this study included pts with RA who initiated ABA or a TNFi, were CCP3+ ( >20 U/mL), SE+ (presence of SE on HLA-DRB1 allele) and had moderate or high CDAI score ( >10) at initiation and 6-mth follow-up (+/− 3 mths). The primary outcome was mean ∆ in CDAI score over 6 mths; secondary outcomes included achievement of remission (CDAI ≤2.8) and low disease activity (CDAI ≤10) and mean ∆ in pain, fatigue and pt global assessment. Analyses were conducted using both propensity score (PS)-trimmed and -matched populations. PS was calculated within each line of therapy. The PS-trimmed cohort included pts with scores overlapping both populations. After PS trimming, TNFi initiators were PS matched 1:1 to ABA initiators within each line of therapy. A sensitivity analysis was conducted in biologic-experienced pts only. Mixed-effects models were used for primary and secondary outcomes and included baseline characteristics that remained unbalanced (standardized difference [sDiff] >0.1) between treatment groups.
Results:
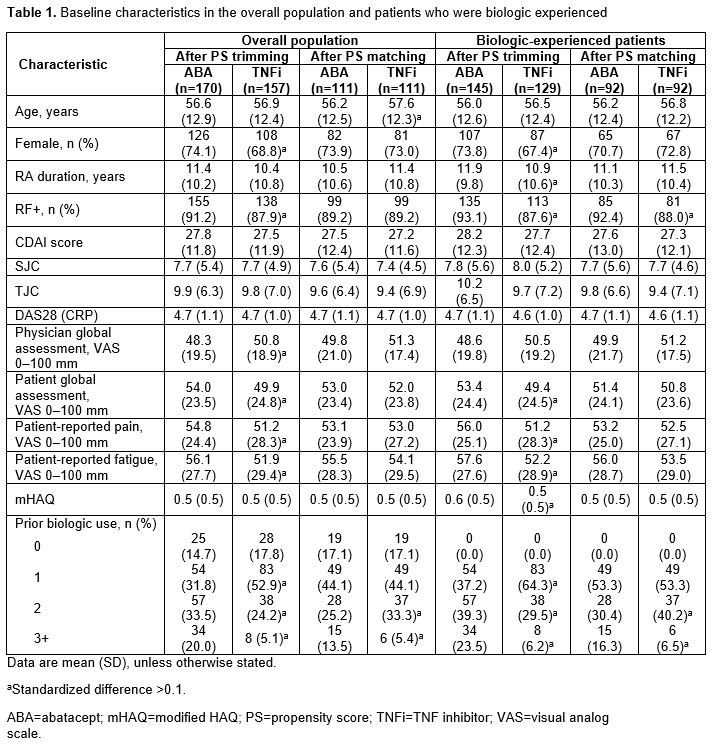
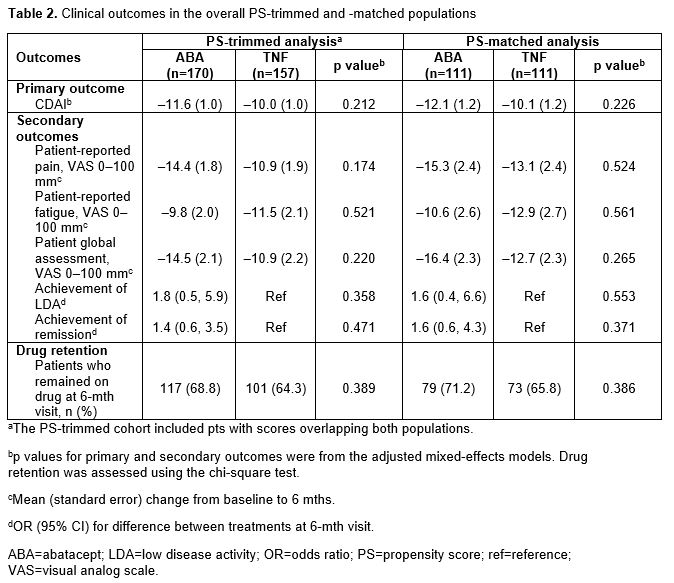
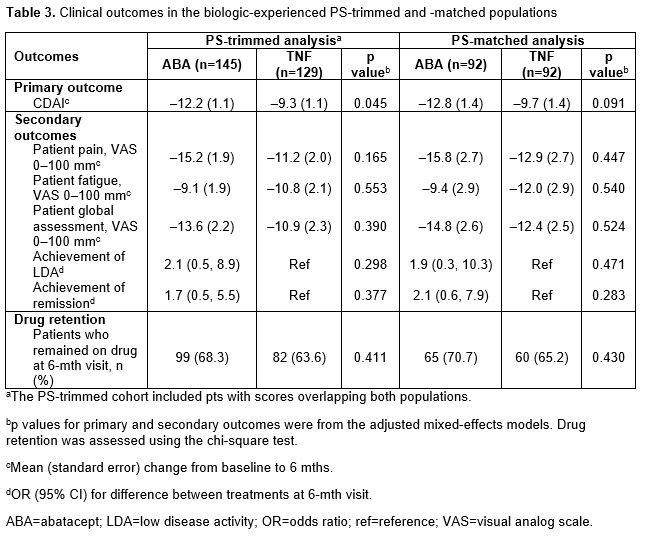
In the overall PS-trimmed cohort (ABA, n=170; TNFi, n=157), baseline characteristics that were imbalanced (sDiff >0.1) between treatments included sex, RF+, physician and pt global assessments, pain, fatigue and prior biologic use (Table 1). Baseline characteristics in the overall PS-matched cohort (ABA, n=111; TNFi, n=111) were generally well balanced. Similar trends for imbalances in baseline characteristics were seen in biologic-experienced cohorts. In the overall population for both the PS-trimmed and -matched cohorts, there were numerically greater improvements in mean ∆ in CDAI and nearly all secondary efficacy outcomes between ABA and TNFi (Table 2). There was no significant difference in the percentage of pts who remained on study medication at 6 mths. Similar trends for efficacy outcomes were seen for biologic-experienced pts; statistical significance was reached for mean ∆ in CDAI in the PS-trimmed cohort (Table 3).
Conclusion: There was a consistent numerical improvement in efficacy outcomes with abatacept over TNFi in pts with long-standing RA who were SE+ and ACPA+ over 6 mths in all four study cohorts. After adjusting for covariates that remained imbalanced between groups in the biologic-experienced PS-trimmed cohort, the improvement in CDAI with abatacept vs TNFi was significant. Additional studies to explore these findings in a larger population would be of interest.
References:
- Huizinga TWJ, et al. Arthritis Rheum 2005;52:3433–3438.
- Rigby W, et al. Presented at EULAR 2019. Poster LB008.
- Pappas DA, et al. BMC Musculoskelet Disord 2014;15:113.
Medical writing: Claire Line, PhD (Caudex)
To cite this abstract in AMA style:
Harrold L, Wittstock K, Kelly S, Han X, Zhuo J, Schrader A, Middaugh N, Moore P, Khaychuk V. The Comparative Effectiveness of Abatacept versus TNF Inhibitors in Patients Who Are ACPA Positive and Have the Shared Epitope: Results from a US National Observational Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-comparative-effectiveness-of-abatacept-versus-tnf-inhibitors-in-patients-who-are-acpa-positive-and-have-the-shared-epitope-results-from-a-us-national-observational-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-comparative-effectiveness-of-abatacept-versus-tnf-inhibitors-in-patients-who-are-acpa-positive-and-have-the-shared-epitope-results-from-a-us-national-observational-study/