Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The Collaborative National Quality and Efficacy Registry (CONQUER) for Scleroderma is a multicenter US-based longitudinal study of patients with systemic sclerosis (SSc) within 5 years of first non-Raynaud’s symptom. Supported by the Scleroderma Research Foundation, CONQUER is designed to provide linked bio-specimen and clinical outcomes data on a longitudinal cohort of SSc patients for validation of hypothesis driven research and to provide a platform for studying patient reported outcomes. The purpose of this analysis was to assess the effectiveness of guideline-based registry practices on database quality at 6 months.
Methods: The CONQUER registry was developed using the guidelines of the International Society for Biological Repositories. It was an iterative process between 13 physicians with an expertise in SSc, patient stakeholders, and information technology experts to ensure there is minimal missing data in 775 fields of data elements.
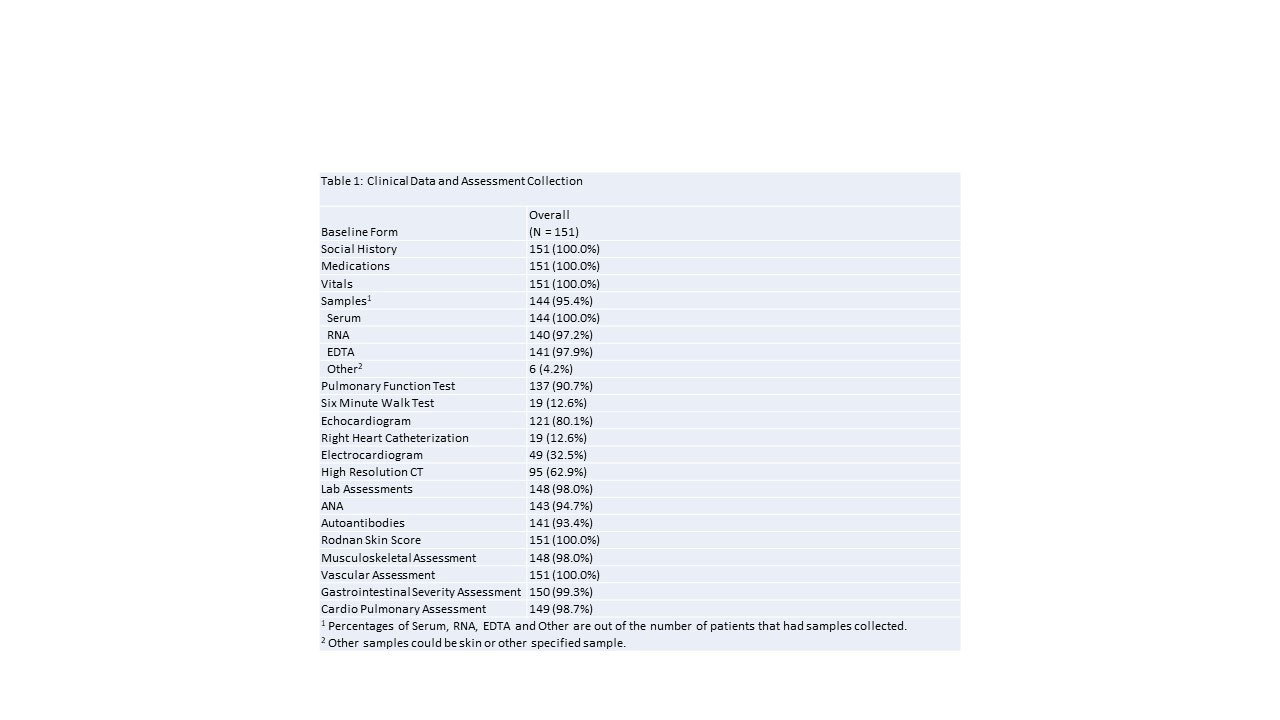
Results: During the first 6 months of the CONQUER Scleroderma study, 151 SSc patients with less than 5 years of disease duration (from first non-Raynaud’s symptom) were recruited. The mean age is 51 ± 14 years, 83% are female and 60% of patients have diffuse disease. Data completion is shown in Table 1. Survey completion rates are above 88% for all patient reported outcome surveys. Bio-specimen collection rates are over 97% and disease severity score completion rates are over 98%.
Conclusion: As demonstrated by data completion, the CONQUER study demonstrates the value of guideline-based registries that are designed by physicians, patients, and information technology experts. CONQUER is a unique and growing resource for studying SSc in a longitudinal, US-based population.
To cite this abstract in AMA style:
Shanmugam V, Frech T, Steen V, Hummers L, Shah A, Bernstein E, Khanna D, Gordon J, Castelino F, Chung L, Hant F, Startup E, VanBuren J, Evnin L, Assassi S. The Collaborative National Quality and Efficacy Registry for Scleroderma: Data Completion Outcomes from a Multicenter United States Cohort Using Guideline-Based Registry Practices [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-collaborative-national-quality-and-efficacy-registry-for-scleroderma-data-completion-outcomes-from-a-multicenter-united-states-cohort-using-guideline-based-registry-practices/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-collaborative-national-quality-and-efficacy-registry-for-scleroderma-data-completion-outcomes-from-a-multicenter-united-states-cohort-using-guideline-based-registry-practices/