Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: To evaluate clinical associations of anti-Th/To antibodies in SSc patients in a multicentre international cohort, focusing on interstitial lung disease (ILD), pulmonary arterial hypertension (PAH), synchronous malignancies, organ damage, mortality.
Methods: A case-control study of prospectively collected data from 23 EUSTAR centres was performed (CP144). For every anti-Th/To+ SSc case, centres provided 2 anti-Th/To- SSc controls matched by sex, age at onset (±5 years) and disease duration (±24 months). ILD functional progression was defined as absolute decline in %pFVC≥5% and/or %pDLCO >10% over 12 months1. Synchronous malignancies were defined as diagnosed within ±2 years from SSc onset2. Organ damage was evaluated through the Scleroderma Clinical Trials Consortium Damage Index (SCTC-DI)3.
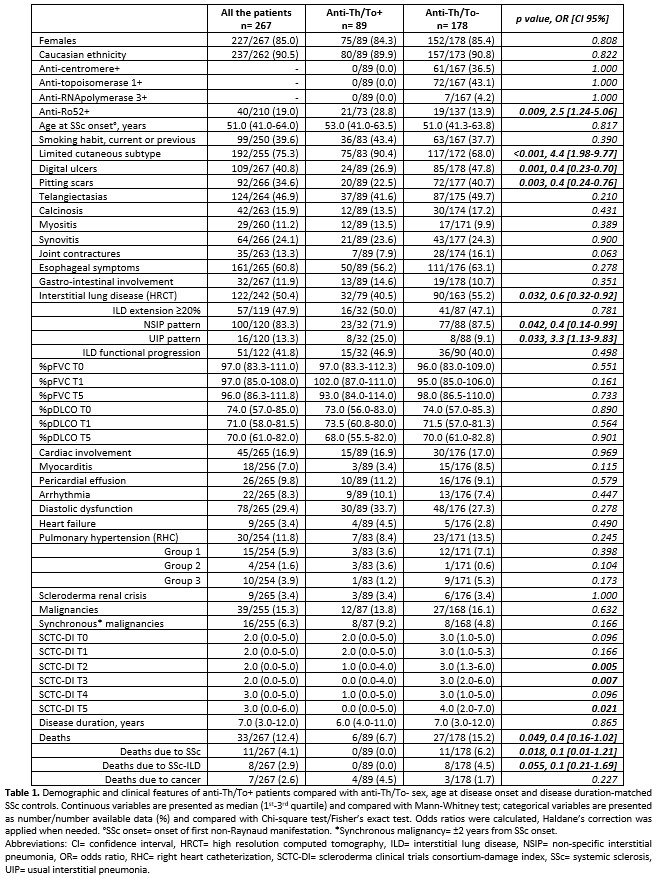
Results: 89 anti-Th/To+ SSc patients, as compared to 178 anti-Th/To- (Table 1), had higher rate of concomitant anti-Ro52 positivity and lower frequency of diffuse cutaneous involvement and digital ulcers/pitting.
ILD was detected in 40.5% anti-Th/To+ patients (vs 55.2% in anti-Th/To-, p:0.032), with non-specific interstitial pneumonia (NSIP) pattern in 71.9%. Functional progression was detected in 46.9% during the first 5 years of disease, similarly to the control group. Only 1 (3.1%) anti-Th/To+ patient developed group 3 PH. A higher prevalence of usual interstitial pneumonia (UIP) pattern was found in cases.
PAH was confirmed at right heart catheterization in 3 (3.6%) anti-Th/To+ cases, slightly less frequently than in controls.
Malignancies rate was similar in the two groups. In anti-Th/To+ cases, the most common synchronous cancer type was breast cancer in 5 cases (2 infiltrating ductal carcinomas, 1 infiltrating lobular carcinoma, 2 not specified), followed by 1 pancreatic, 1 thyroid 1 cecum cancer.
Anti-Th/To+ patients accrued less organ damage as compared to controls, exhibiting lower DI scores after 2, 3 and 5 years from SSc onset.
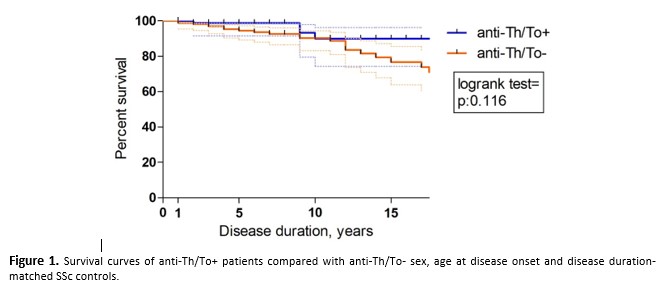
6 (6.7%) anti-Th/To+ patients died at 79.5 (72.5-84.3) years of age and 9.5 (9.0-17.5) years of disease duration with no deaths attributable to SSc-ILD or other SSc complications. Survival curves are depicted in Figure 1: anti-Th/To+ patients had a survival rate of 100.0% at 1 year, 98.8% at 5 years, and 89.9% at both 10 and 15 years of disease duration, slightly higher than controls (98.8% at 1, 94.5% at 5, 90.3% at 10 and 76.7% at 15 years of disease duration).
Conclusion: Through the analysis of this multicentric series of anti-Th/To+ SSc patients, well known clinical associations were confirmed in particular limited cutaneous involvement in most cases and ILD in half of the cases. PAH seems to be part of anti-Th/To spectrum only in a small percentage of patients while the relationship with synchronous malignancies needs to be better explained in future analyses. Globally, it seems that anti-Th/To+ patients have favorable outcome characterized by mild organ damage and good survival.
References: 1Raghu G. 2022;2Lazzaroni MG. 2017;3Ferdowsi N. 2019
To cite this abstract in AMA style:
Moschetti L, Pedretti E, Bonomi F, Martín López M, Cacciapaglia F, sieiro santos c, Moroncini G, Allanore Y, Caetano J, Granel B, Groseanu L, De Santis M, Kuwana M, Codullo V, Pereira Silva M, Bearzi P, Belloli L, Taberner-Cortés A, Maglio C, Del Galdo F, Campochiaro C, Truchetet M, Cuomo G, Parvu M, Iannone F, Carreira P, Guiducci S, Franceschini F, Airò P, Lazzaroni M. The Clinical Phenotype of Anti-Th/To+ Patients in Systemic Sclerosis: A Case-control Study Within the European Scleroderma Trials and Research (EUSTAR) Cohort [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-clinical-phenotype-of-anti-th-to-patients-in-systemic-sclerosis-a-case-control-study-within-the-european-scleroderma-trials-and-research-eustar-cohort/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-clinical-phenotype-of-anti-th-to-patients-in-systemic-sclerosis-a-case-control-study-within-the-european-scleroderma-trials-and-research-eustar-cohort/