Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The aim of this study was to develop and validate new classification criteria for Anti-Synthetase Syndrome (ASSD) using data and consensus driven methodologies and global participation.
Methods: A literature review identified features used to define ASSD and develop initial variable list, which was used for data collection of cases and controls.
Two distinct but complementary approaches were adopted to develop new candidate criteria: data-driven analysis of patient information and multi-criteria decision analysis (MCDA) under steering committee (SC) involving expert rheumatologists, dermatologists, neurologists, and pulmonologists worldwide.
Data on ASSD cases and controls (mimickers of ASSD) were collected through RedCap database. Patient information included demographics, symptoms, laboratory tests, and anti-synthetase antibody results. A preliminary univariate analysis was conducted to inform a multivariate analysis resulting in the data-driven candidate model using derivation dataset followed by internal validation. The MCDA method involved ranking and scoring the importance of different clinical features for ASSD classification, resulting in weighted score-based criteria followed by internal validation on clinical vignette.
Patients’ serum was tested for anti-synthetase antibodies using a central gold standard method to develop a Gold standard dataset for validation. Cases and controls not used for derivation developed an independent validation cohort.
The experts selected the best model through a Consensus Conference performed with Nominal Group Technique.
Results: CLASS project database included 120 variables collected on cases (N=1952) and controls (N=2097), with 19% of patients centrally tested for antibodies. A SC comprising of 20 prominent myositis experts, identified one and three candidate models from the data-driven and the MCDA approaches, respectively.
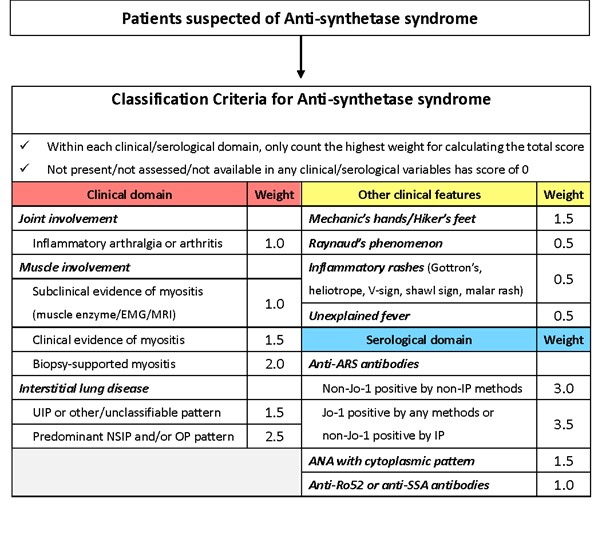
After discussion and refinement, consensus was developed on a final set of criteria based on its face validity, ease of use, feasibility and performance in both MCDA-based clinical vignettes and real-world data (Fig. 1).
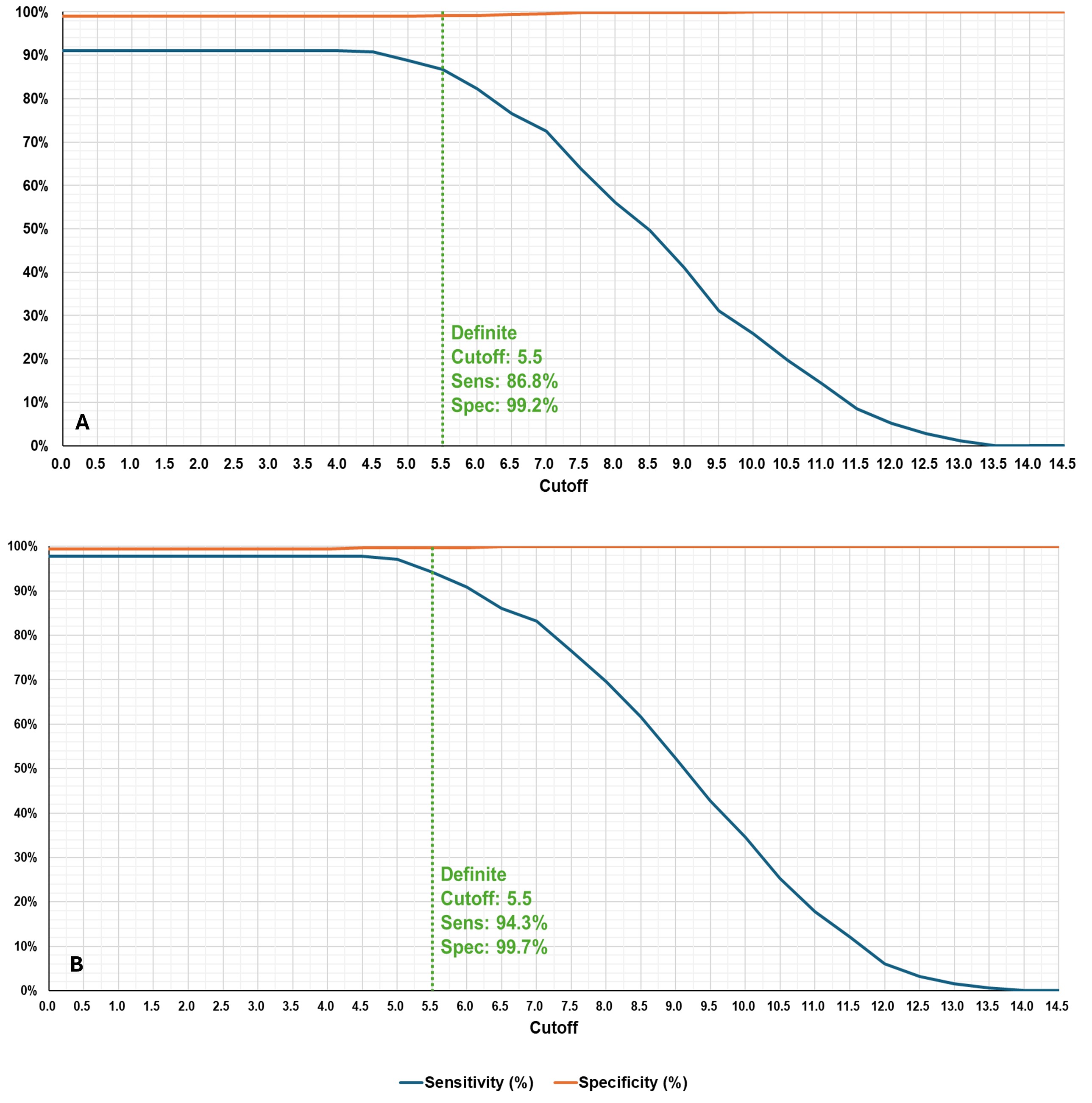
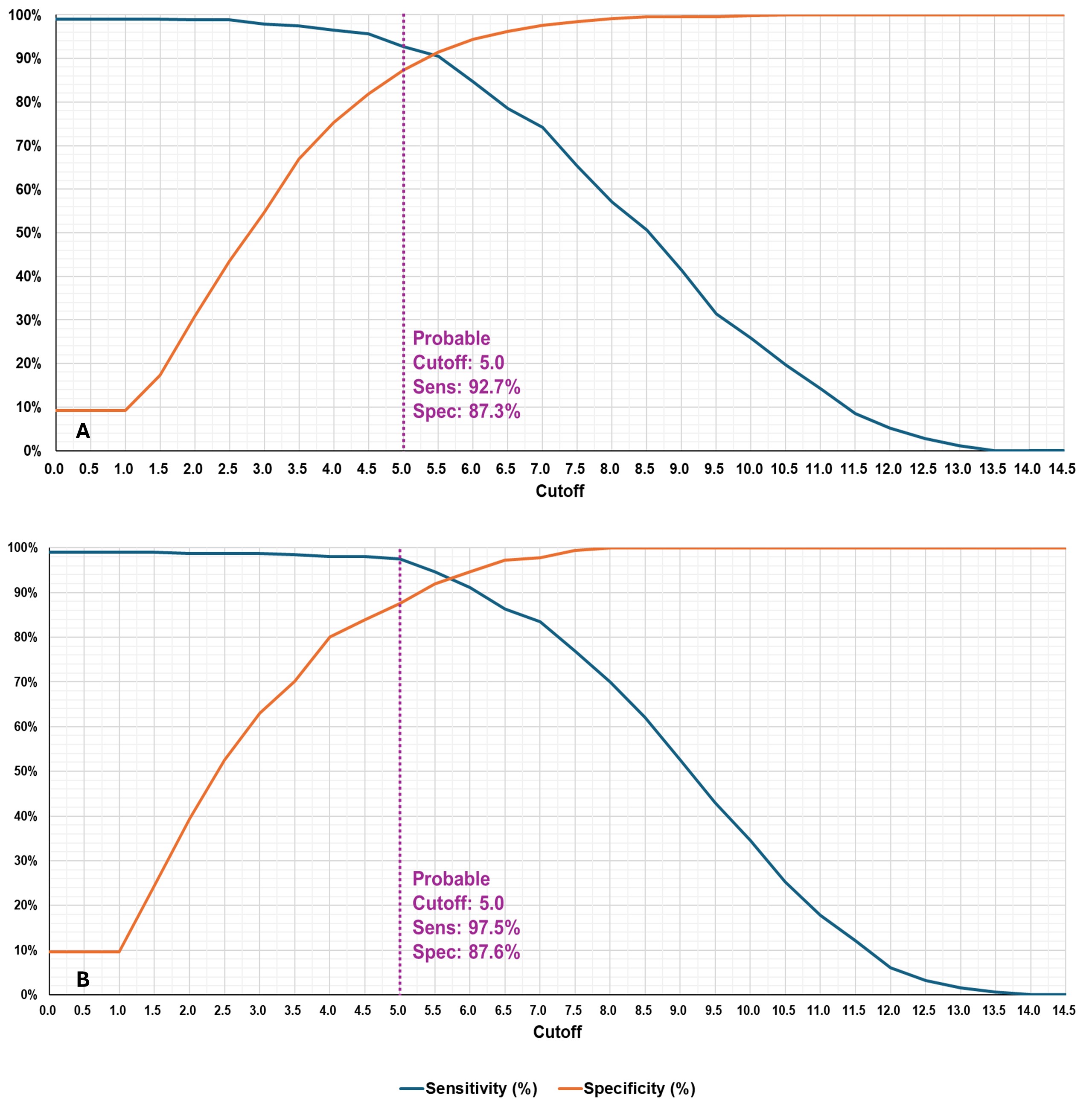
The final criteria define two levels of classification: Probable and Definite ASSD. Both levels require the presence of at least one core clinical feature (arthritis, myositis, or lung disease) and assign points based on additional features and anti-synthetase antibody status. In addition, definite ASSD requires confirmation by anti-synthetase antibody testing. The criteria for Definite ASSD displayed a sensitivity/specificity of 86.8/99.2% on the independent validation dataset and 94.3/99.7% on the gold standard dataset. The criteria for Probable ASSD displayed a sensitivity/specificity of 92.7/87.3% on the independent validation dataset and 97.5/87.5% on the gold standard dataset (Fig. 2 and 3).
Conclusion: Novel, data and consensus driven classification criteria for ASSD have been established with excellent validity, feasibility and global consensus. These criteria should improve the consistency and accuracy of ASSD classification across the world and facilitate future research.
The project was funded by ACR/EULAR and ASSD criteria will be submitted for their approval.
To cite this abstract in AMA style:
Zanframundo G, Faghihi Kashani S, Yoshida A, Dourado E, Bauer Ventura I, Rivero Gallegos D, Loganathan A, Sambataro G, Bozan F, Bae S, Lim D, Cheli M, Yamano Y, Bonella F, Corte T, Doyle T, Fiorentino D, Gonzalez-Gay M, Hudson M, Kuwana M, Lundberg I, Mammen A, McHugh N, Miller F, Montecucco C, Oddis C, Rojas-Serrano J, Schmidt J, Scirè C, Selva-O'Callaghan A, Werth V, Rozza D, Morosini M, Bozzini S, Cavagna L, Aggarwal R. The Classification Criteria for Anti-Synthetase Syndrome (Class) Project [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-classification-criteria-for-anti-synthetase-syndrome-class-project/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-classification-criteria-for-anti-synthetase-syndrome-class-project/