Session Information
Date: Monday, November 9, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Bench to Bedside
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Type I IFN contributes to SLE pathogenesis and stimulates the kynurenine/tryptophan (KYN/TRP) pathway, producing elevated quinolinic acid (QA) levels relative to kynurenic acid (KA) and thus creating a potential neurotoxic imbalance. We found that SLE subjects have elevated KYN/TRP and QA/KA ratios vs. healthy controls (HC), and that the QA/KA ratio correlated with poor working memory performance after controlling for other factors (submitted abstract). We hypothesized that peripheral blood interferon stimulated gene (ISG) expression would associate with QA/KA ratios in these same subjects.
Methods: We measured ISG expression (whole blood RNA sequencing) and serum levels of KYN, TRP, QA, and KA (HPLC) in 72 SLE subjects and 73 HC. ISG were identified based on published gene sets, and the Interferome database.[1, 2] IFN scores were derived for each subject to quantify individual ISG expression and analyze associations with metabolite ratios. Since it is unknown which ISG contribute most to an “IFN signature,” we used 4 approaches to define IFN scores (Table 2). Expression was measured by fold change (FC) relative to HC, a point system for FC, or z-scores. Differences in characteristics and metabolite ratios in SLE vs. HC were assessed (t-test, Mann-Whitney, or chi square), and Spearman’s correlations were determined between metabolite ratios and IFN scores among SLE subjects; significance was set at p< 0.05.
Results: There were no demographic differences between SLE and HC, and SLE subjects had higher median metabolite ratios (Table 1). There were 933 genes that had ≥2-fold differential expression in SLE vs. HC (p< 0.05). Of 110 ISG reported by Arazi et al, 108 had ≥2-fold higher expression in SLE vs. HC. Unsupervised principal component analysis (PCA) distinguished SLE from HC (Figure 1); of the top 100 most variant genes, 70 were ISG. IFN scores, based on the first 3 objective approaches, correlated with KYN/TRP but not with QA/KA ratios (Table 2). However, 82 of the 933 differentially expressed genes correlated with QA/KA ratios (p< 0.05), and 38 (46%) were ISG; 31/38 (82%) of these were type I responsive ISG. A Targeted IFN score (approach 4a) using these 38 ISG correlated with QA/KA and KYN/TRP ratios (Table 2). A separate Targeted-Modular IFN score comprised of 3 type I ISG (CCL8, CXCL10, LAP3) included in published ISG sets and associated with neuroinflammation, also correlated with both ratios (Table 2, 4b).
Conclusion: This is the first study in SLE subjects to demonstrate an association between ISG expression, KYN/TRP pathway activation, and an elevated QA/KA ratio. The targeted IFN scores need validation in a separate cohort to justify their utility as a biomarker for IFN-mediated KYN/TRP pathway activation, and their potential use in measuring success of anti-IFN therapies in SLE to ameliorate a potential neurotoxic QA/KA imbalance.
References:
- Arazi, A., et al., The immune cell landscape in kidneys of patients with lupus nephritis. Nat Immunol, 2019. 20(7): p. 902-914.
- Chiche, L., et al., Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol, 2014. 66(6): p. 1583-95.
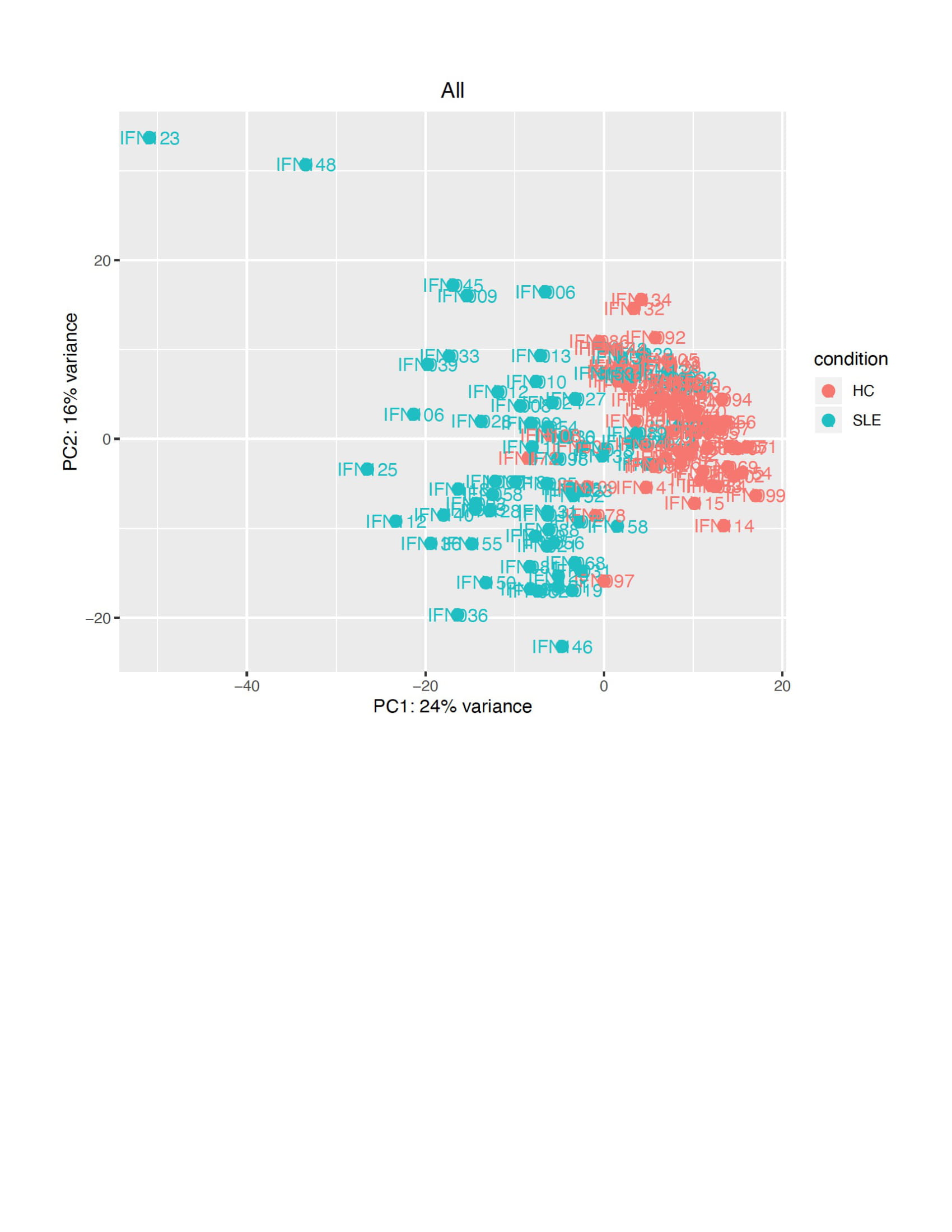 Figure 1: Principal component analysis (PCA) of gene expression in SLE compared to healthy controls (HC). PCA conducted on DESeq2 normalized and variance stabilized gene expression values using the function prcomp in R version 3.5 is displayed[1]. The default top 500 most highly variant genes were selected. 1. Kolde, R., 2012 Package ‘pheatmap’. Bioconductor 1–6. Available at: https://CRAN.R-project.org/package=pheatmap
Figure 1: Principal component analysis (PCA) of gene expression in SLE compared to healthy controls (HC). PCA conducted on DESeq2 normalized and variance stabilized gene expression values using the function prcomp in R version 3.5 is displayed[1]. The default top 500 most highly variant genes were selected. 1. Kolde, R., 2012 Package ‘pheatmap’. Bioconductor 1–6. Available at: https://CRAN.R-project.org/package=pheatmap
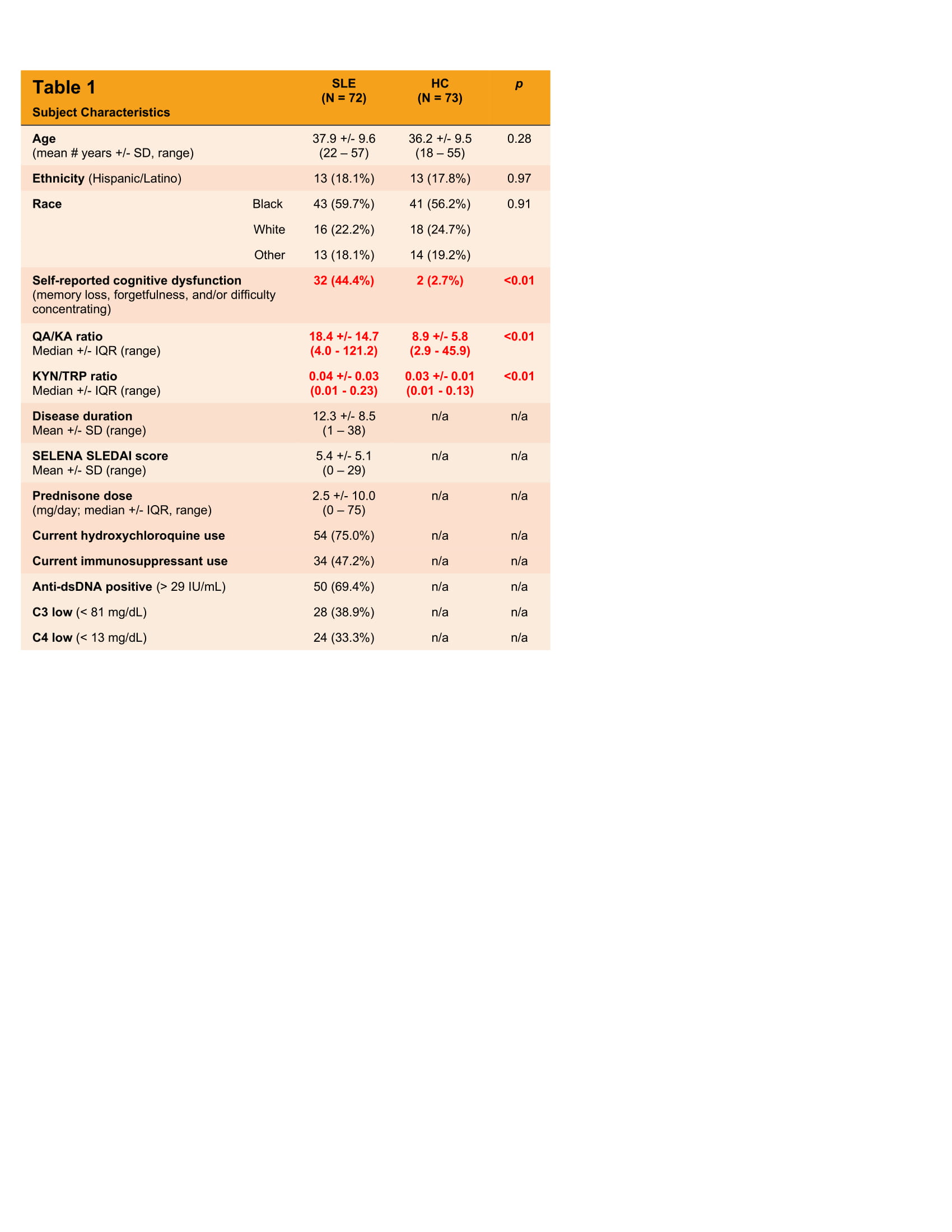 Table 1: SLE subject and healthy control (HC) characteristics and KYN/TRP pathway metabolite ratios. All data is reported either as a mean (or median where indicated) +/- standard deviation (or interquartile range), or as a frequency (%). All data refers to that which was collected at the time of evaluation.
Table 1: SLE subject and healthy control (HC) characteristics and KYN/TRP pathway metabolite ratios. All data is reported either as a mean (or median where indicated) +/- standard deviation (or interquartile range), or as a frequency (%). All data refers to that which was collected at the time of evaluation.
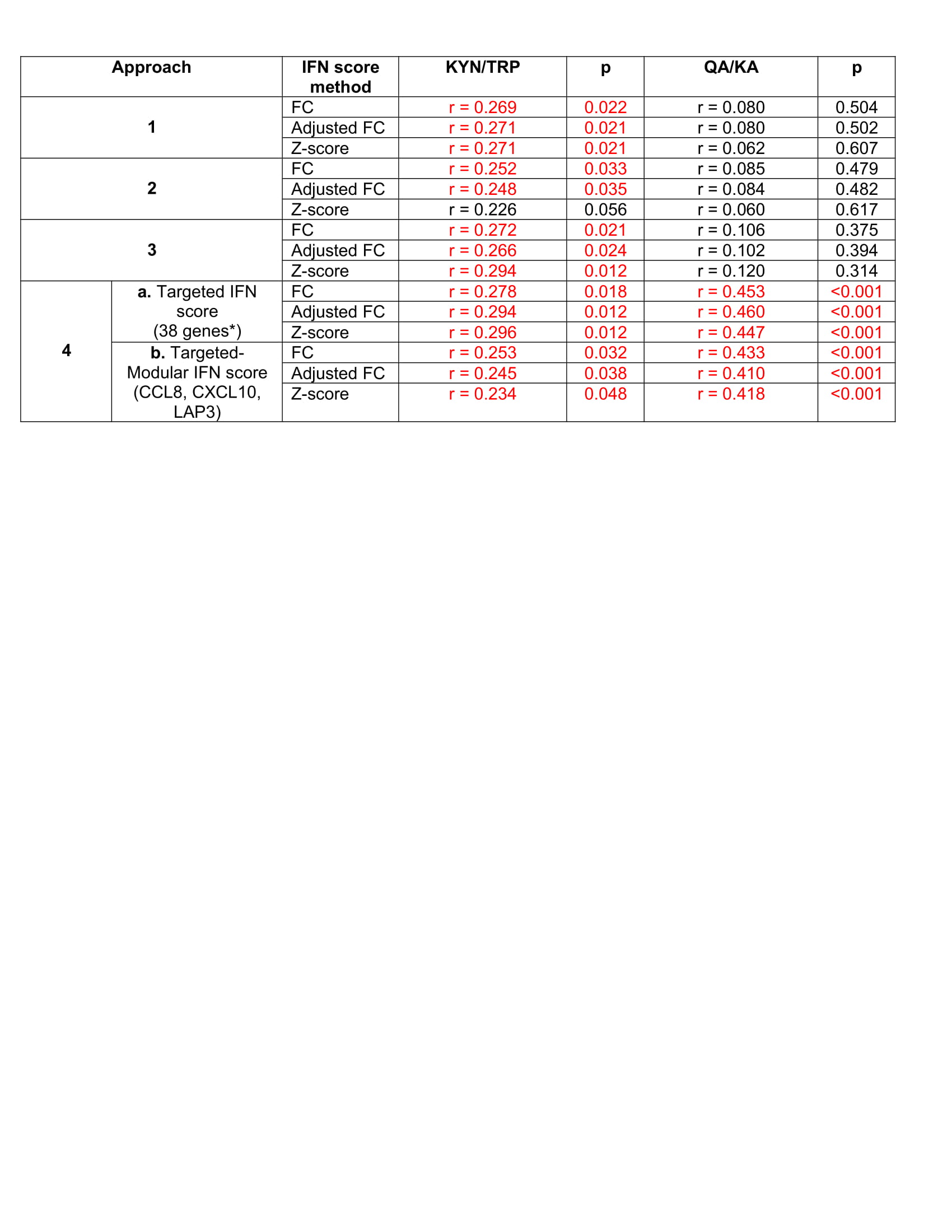 Table 2: Correlations Between IFN Scores, Defined by 4 Approaches, and KYN/TRP Pathway Metabolite Ratios in SLE subjects. Approach 1 derived an IFN score from the 70 ISG that were in the top 100 most variably expressed genes between SLE and HC determined by PCA, Approach 2 included all of the 110 ISG reported by Arazi et al in their single cell RNA sequencing analysis of IFN response in infiltrating cells in lupus nephritis, and Approach 3 included 19 ISG from the 110 ISG included in Approach 2 that are more responsive to type I IFN than type II IFN, according to the Chiche et al modules of co-expressed ISG. Approach 4a included ISG that had significant, positive correlations with the QA/KA ratio, and 4b included 3 ISG from 4a that were also included in the 110 Arazi ISG set and in the Chiche modules and are associated with neuroinflammation.[1, 2] Scores were computed using fold change (FC) relative to HC, adjusted FC (point system) and z-scores. Spearman’s correlation coefficients (r) are shown between each IFN score (grouped by approach, and the method used to compute the score) and the metabolite ratios. Significant correlations are highlighted in red. *Refers to the 38 ISG that had a significant, positive correlation with the QA/KA ratio in SLE subjects 1. van Weering, H.R., et al., CXCL10/CXCR3 signaling in glia cells differentially affects NMDA-induced cell death in CA and DG neurons of the mouse hippocampus. Hippocampus, 2011. 21(2): p. 220-32. 2. Banisor, I., et al., Involvement of beta-chemokines in the development of inflammatory demyelination. J Neuroinflammation, 2005. 2(1): p. 7.
Table 2: Correlations Between IFN Scores, Defined by 4 Approaches, and KYN/TRP Pathway Metabolite Ratios in SLE subjects. Approach 1 derived an IFN score from the 70 ISG that were in the top 100 most variably expressed genes between SLE and HC determined by PCA, Approach 2 included all of the 110 ISG reported by Arazi et al in their single cell RNA sequencing analysis of IFN response in infiltrating cells in lupus nephritis, and Approach 3 included 19 ISG from the 110 ISG included in Approach 2 that are more responsive to type I IFN than type II IFN, according to the Chiche et al modules of co-expressed ISG. Approach 4a included ISG that had significant, positive correlations with the QA/KA ratio, and 4b included 3 ISG from 4a that were also included in the 110 Arazi ISG set and in the Chiche modules and are associated with neuroinflammation.[1, 2] Scores were computed using fold change (FC) relative to HC, adjusted FC (point system) and z-scores. Spearman’s correlation coefficients (r) are shown between each IFN score (grouped by approach, and the method used to compute the score) and the metabolite ratios. Significant correlations are highlighted in red. *Refers to the 38 ISG that had a significant, positive correlation with the QA/KA ratio in SLE subjects 1. van Weering, H.R., et al., CXCL10/CXCR3 signaling in glia cells differentially affects NMDA-induced cell death in CA and DG neurons of the mouse hippocampus. Hippocampus, 2011. 21(2): p. 220-32. 2. Banisor, I., et al., Involvement of beta-chemokines in the development of inflammatory demyelination. J Neuroinflammation, 2005. 2(1): p. 7.
To cite this abstract in AMA style:
Anderson E, Jin Y, Goodwin S, Roeser J, Furie R, Aranow C, Volpe B, Diamond B, Mackay M. The Association of Interferon-α with Kynurenine/Tryptophan Pathway Activation in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/the-association-of-interferon-%ce%b1-with-kynurenine-tryptophan-pathway-activation-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-association-of-interferon-%ce%b1-with-kynurenine-tryptophan-pathway-activation-in-systemic-lupus-erythematosus/
