Session Information
Date: Monday, November 11, 2019
Title: 4M096: Spondyloarthritis Including Psoriatic Arthritis – Clinical III: Miscellaneous (1818–1823)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Currently the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Spinal pain are used to assess whether a patient with Axial Spondyloarthritis (AxSpA) requires biologic therapy and their response to biologic therapy. However, the BASDAI does not just reflect inflammatory disease activity as it includes questions relating to fatigue (question 1) and musculoskeletal areas of tenderness (question 4) and therefore escalating treatment to biologic therapy, and/or stopping or switching biologic treatment based on a BASDAI may not improve symptoms or quality of life. We assessed the sensitivity of individual questions of the BASDAI, ASDAS and CRP against the response to biologics.
Methods: Data from 85 AxSpA patients were collected, including demographic characteristics, extraarticular manifestations and outcome measures. Patients were then analysed according to the following groupings: responder or non-responder to biologics. In each group, mean scores for individual questions of BASDAI, BASFI, ASDAS and CRP were calculated. Subjects were further analysed according to groups: Group 1 (BASDAI ≥4, ASDAS ≥2.1), Group 2 (BASDAI < 4, ASDAS ≥2.1) and Group 3 (BASDAI < 4, ASDAS < 2.1).
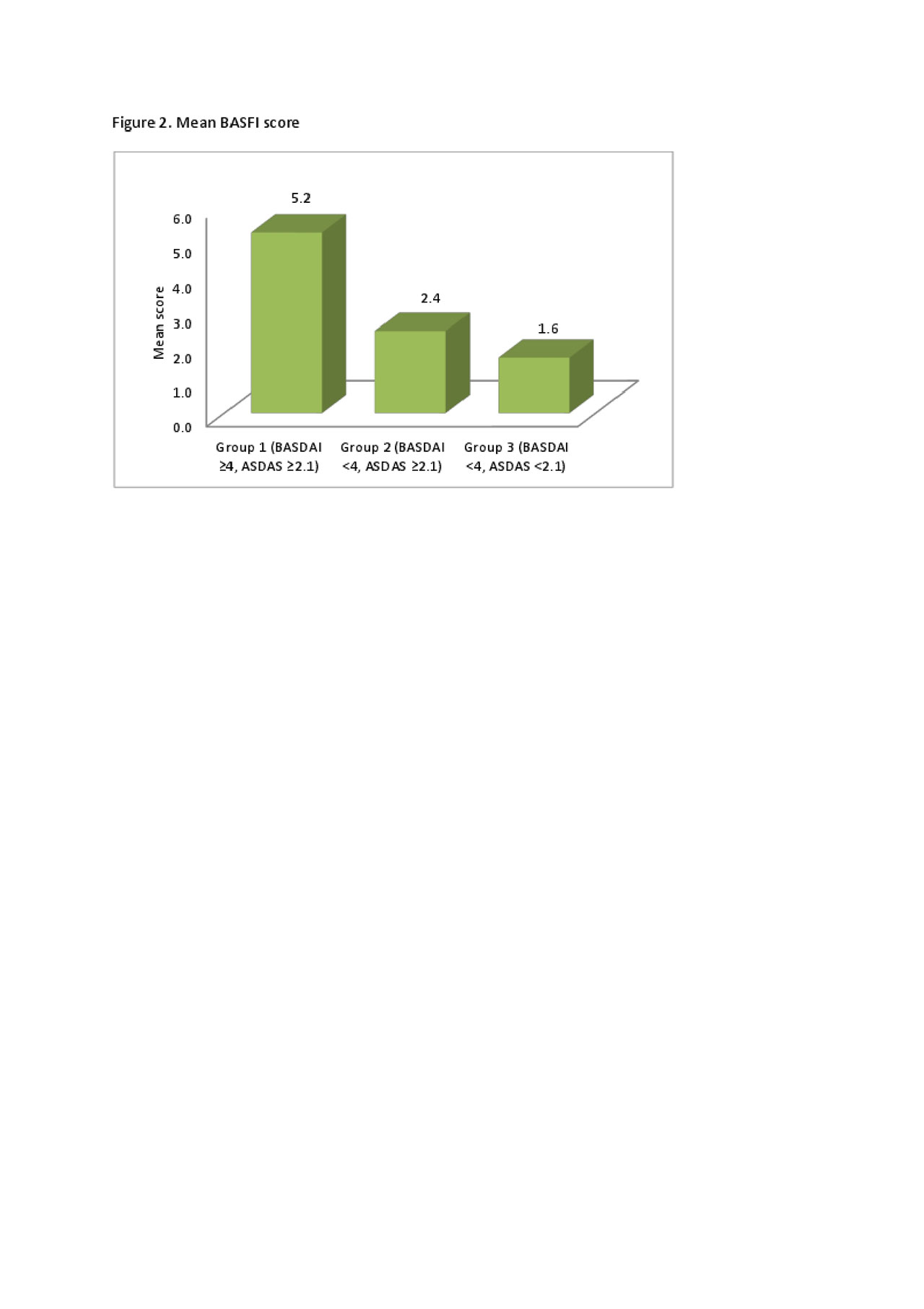
Results: There were n=65 male and n=20 female subjects. Mean (SD) age was 44.7 (13.3) years and AxSpA disease duration 9.5 (9.9) years. The mean BASDAI scores at baseline in patients receiving biologics were similar in responders 6.1 (1.8) and non-responders 6.2 (1.4). Post treatment with biologics, clinical non-responders had a higher mean score for Q1 and Q4 of the BASDAI (7.7 and 6.5) compared to responders (3.8 and 2.2). A similar trend was seen for BASFI (mean 5.9 in non-responders compared to 2.2 in responders). Further analysis using ASDAS, showed that patients in Group 1 had higher scores for Q1 and Q4 of the BASDAI (mean 6.9 and 6.0 respectively) and coexistent fibromyalgia compared to Groups 2 (mean 2.6 and 2.6) and 3 (mean 3.3 and 1.7), see Figure 1. This correlated with a high BASFI (mean of 5.2 in Group 1) compared to Group 2 (2.4) and 3 (1.6), see Figure 2. Patients in Group 2 had a higher CRP (mean 11.4) than the other groups (3.5 in Group 1 and 3.1 in Group 3), reflecting higher inflammatory burden. There was a lower proportion of females (16%) in Group 3 compared to the other groups (32% in Group 1 and 30% in Group 2). Furthermore, Group 3 had the highest percentage of patients with extra-articular manifestations (73%, compared to 51% and 58% respectively for Groups 1 and 2).
Conclusion: These findings support the hypothesis that fatigue and pain, as reflected by high scores for Q1 and Q4 of the BASDAI and coexistent fibromyalgia, are possible drivers of high perceived disease activity and non-response to biologics. This is correlated with a high BASFI. The elevated CRP seen in Group 2 suggests a higher burden of inflammation, in spite of low BASDAI scores, suggesting a need for more intensive therapy in this group. There was a trend for biologic responders to be males and with more extra-articular manifestions. ASDAS provides a means of incorporating more objective measures of disease activity, such as CRP, into clinical assessment, and therefore provides a useful adjunct to traditional Bath scores in assessing response to biologics.
To cite this abstract in AMA style:
Sacks S, Rigler K, Chan A. The Ankylosing Spondylitis Disease Activity Score Reflects and Predicts Response to Biologic Treatment in Axial Spondyloarthritis Patients with Coexistent Fibromyalgia Compared to the Bath Ankylosing Spondylitis Disease Activity Index [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-ankylosing-spondylitis-disease-activity-score-reflects-and-predicts-response-to-biologic-treatment-in-axial-spondyloarthritis-patients-with-coexistent-fibromyalgia-compared-to-the-bath-ankylosing/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-ankylosing-spondylitis-disease-activity-score-reflects-and-predicts-response-to-biologic-treatment-in-axial-spondyloarthritis-patients-with-coexistent-fibromyalgia-compared-to-the-bath-ankylosing/