Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Isolated anti-Ro52 antibodies (anti-Ro52) influence the ACR/EULAR 2016 classification criteria of Sjögren’s Disease (SD), meaning that patients with isolated anti-Ro52 positivity and abnormal salivary or lachrymal functional tests may fulfill the criteria avoiding a labial gland biopsy (LSGB). However, anti-Ro52 can be detected in patients with a broad spectrum of other autoimmune disorders; therefore, their role in SD classification remains a matter of debate. We hypothesize that anti-Ro52 levels might be associated with a positive LSGB. The purpose of this study was therefore: 1. To investigate the correlation between anti-Ro52 titers and histological findings in patients with suspected SD consecutively undergoing LSGB. 2. To analyze the correlation between anti-Ro52 levels and SD-related disease features in the subgroup of patients with isolated anti-Ro52 positivity.
Methods: Consecutive patients undergoing an LSGB for suspected SD were included. Clinical and histological data were collected. Serum from each patient was tested for anti-Ro52, and anti-Ro60, with commercially available immunoblot, thus analyzing the quantitative titer of the autoantibodies with band densitometry. Patients were divided into three groups according to anti-Ro52 above the third and below the first percentile. Descriptive statistics were performed with SPSS software.
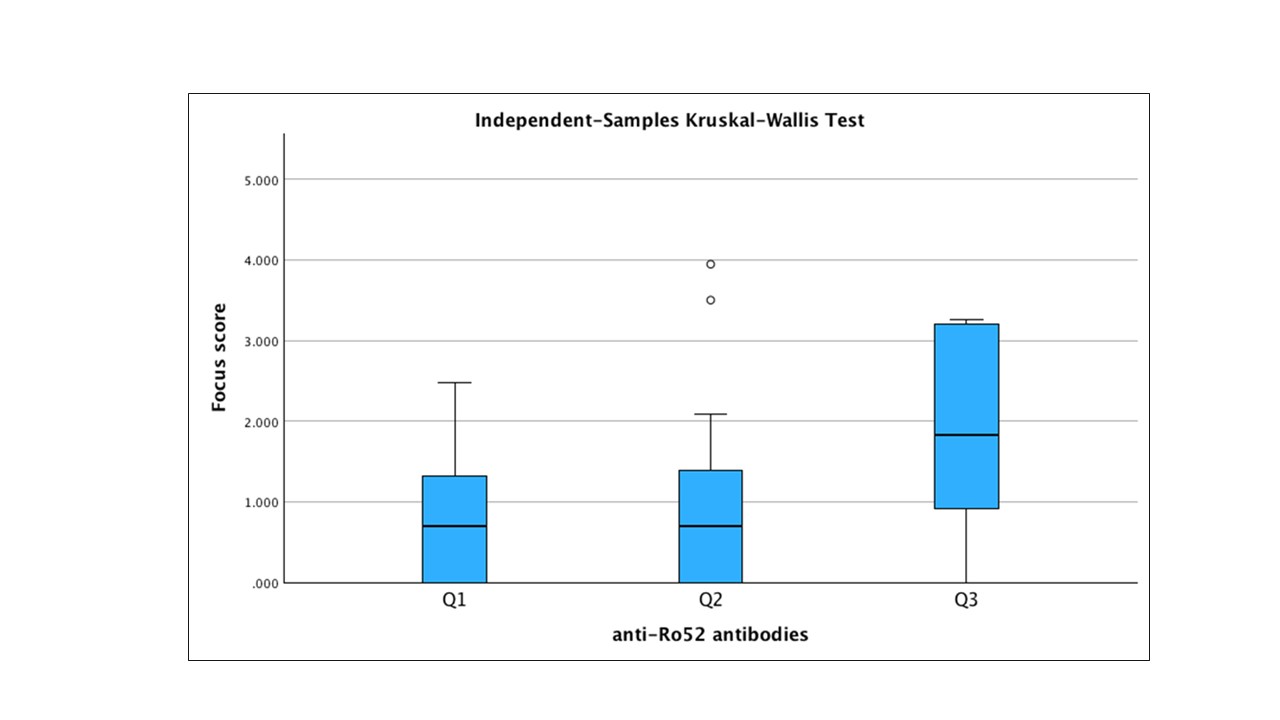
Results: We included 175 (F:M=160:15) patients, median age 54 (IQR 45-64). The majority (88/175, 50.3%) presented a double positivity for Ro60/Ro52, 27/175 (15.4%) an isolated positivity for anti-Ro60 and 60/175 (34.3%) an isolated positivity for anti-Ro52. The diagnosis of SD was confirmed in 147/175 patients. Titers of anti Ro-52 significantly correlated with anti-Ro60 (r=0.358, p< 0.001) and anti-SSB titers (r=0.364, p< 0.001) as well as with LSG FS (r=460, p< 0.001), number of foci (p< 0.404, p< 0.001) and GC (r=0.364, p< 0.001) and SGUS score. Moreover, anti-Ro52 titer fairly correlated with RF (r=0.268, p< 0.01), IgG (r=0.301, p< 0.001), and ESSDAI score (r=0.281, p< 0.001) and inversely with lymphocytes count (r=-0.171, p< 0.05) and C4 (r=-0.233, p< 0.01). No correlation was found between anti-Ro-52 levels, sicca symptoms, ESSPRI and the results of either the oral or ocular tests. When we limited the analysis to the 60/175 patients with isolated Ro52, those 12/60 (20%) with anti-Ro52 titer above the third percentile satisfied the 2016 ACR/EULAR classification criteria for SD in all the cases, presented the highest FS (p< 0.01) (FIG 1), numbers of foci (p< 0.01) and presence of GCs (p< 0.05) and showed more frequently an OMERACT SGUS≥2 (p< 0.05) with respect to the rest of the patients with isolated Ro-52.
Conclusion: This study suggests that anti-Ro52 titer seems to correlate with LSG histology and may help to identify a more homogeneous subset of patient with more severe glandular inflammation among subjects with isolated Ro52. Further studies are warranted to establish whether anti-Ro52 should be separately considered in SD classification criteria.
To cite this abstract in AMA style:
Fonzetti S, Porciani C, Donati V, Testa D, Fulvio G, La Rocca G, Navarro García I, Ferro F, Mosca M, Migliorini P, Baldini C. The Added Value of anti-Ro52 Antibody Titer in the Diagnosis of Sjögren’s Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-added-value-of-anti-ro52-antibody-titer-in-the-diagnosis-of-sjogrens-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-added-value-of-anti-ro52-antibody-titer-in-the-diagnosis-of-sjogrens-disease/