Session Information
Date: Monday, November 9, 2020
Title: RA – Treatments II: Potential Harms & Adverse Events (1998–2002)
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Although glucocorticoids (GC) remain an essential part of the therapeutic strategy in Rheumatoid Arthritis (RA), the long-term risk of adverse events of low-dose GC treatment is still controversial.
Methods: We analysed data from the early arthritis (less than 6 months’ disease duration) ESPOIR cohort. Patients were stratified in two groups, with or without GC treatment at least once during their follow-up (median 10 years IQR [9-10]). The primary outcome was a composite of death, cardiovascular diseases (CVD) (including myocardial ischemia, cerebrovascular accident and heart failure), severe infection and fracture. In order to reduce the impact of treatment selection bias and potential confounding factors, the weighted Cox time-dependent analysis model was used with inverse probability of treatment weighting (IPTW) propensity score method.
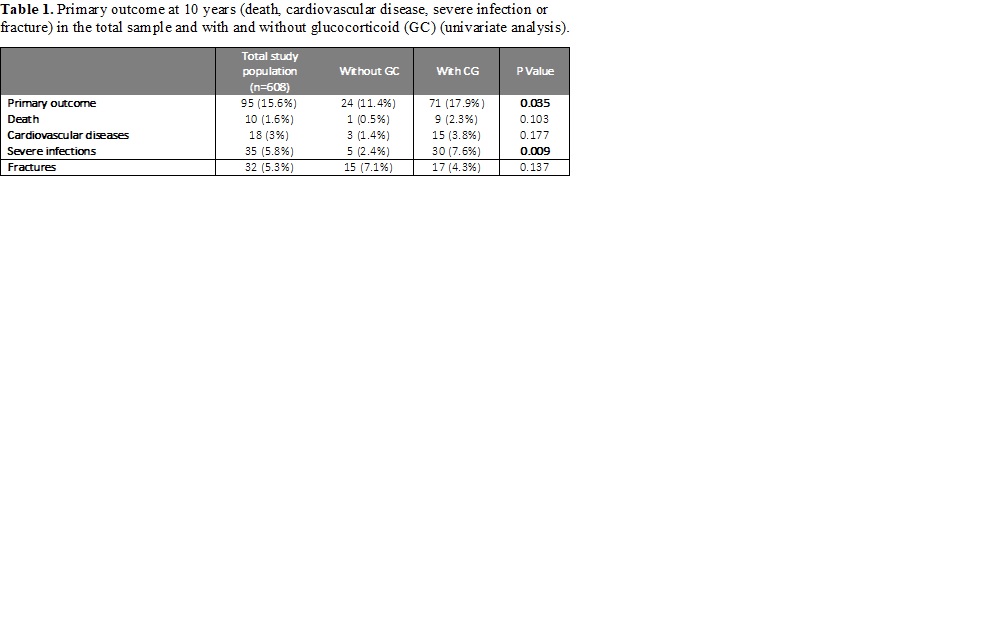
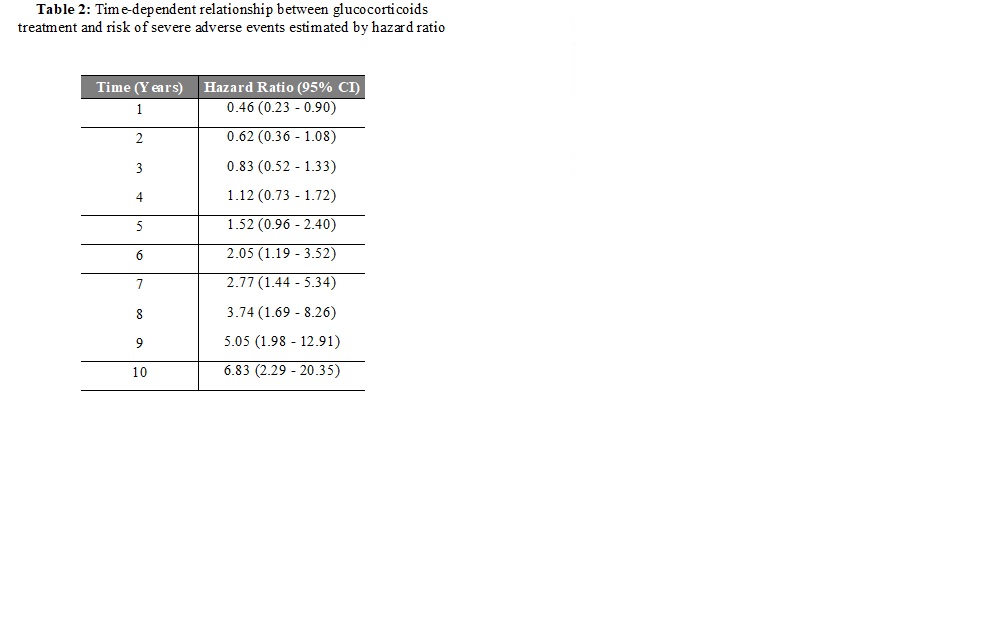
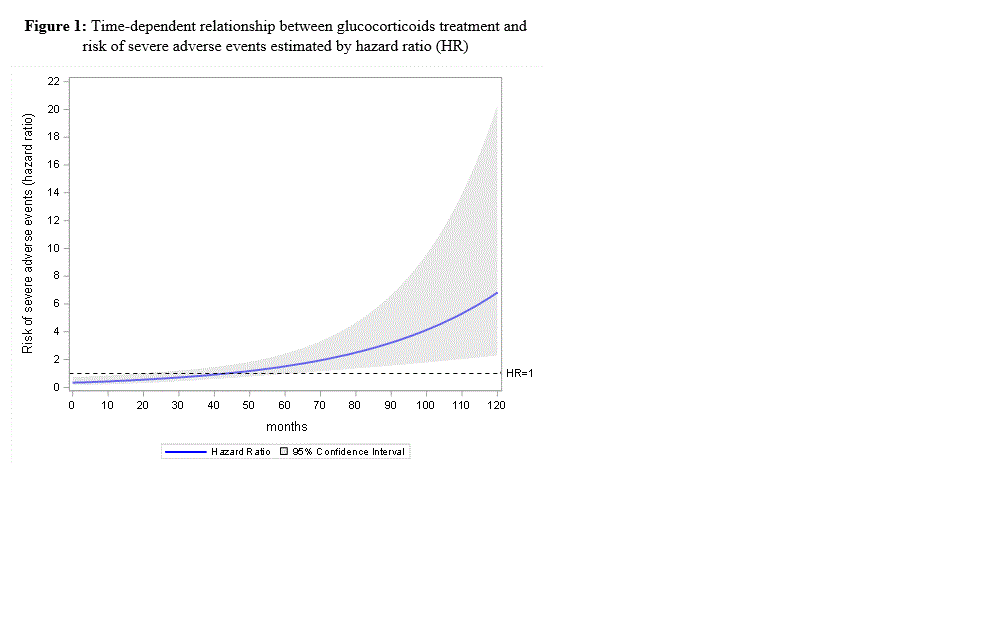
Results: Among the 608 RA patients (480 women, mean age of 47.5 ± 12.1 years), 397 patients (65%) received low-dose prednisone (mean 2.8 ±2.8 mg/d, median 1.9 mg/day [IQR 0.6-4.2], mean cumulative GC dose of 8468 mg ±8376), mainly during the first 6 months (70%). The mean duration of GC treatment was 44.6 months ± 40.1. Overall, 95 events were identified during the follow-up: 10 deaths, 18 CVD, 32 fractures and 35 severe infections. Based on univariate analysis at 10 years, patients taking GC experienced significantly more events (n=71) than those without GC (n=24) (p=0.035) (table1). Interestingly, we evidenced a significant cumulative dose-effect (p=0.007), particularly for the risk of severe infections (p=0.024) and CVD (p=0.001). On weighted Cox time-dependent analysis, using the IPTW propensity score method, the risk of events over time was significantly associated with GC treatment (p < 0.001), age, history of hypertension and erythrocyte sedimentation rate (figure1). The risk associated with GC treatment, estimated by the hazard ratio (HR), increased between the first follow-up visit (HR at 6 months = 0.39, 95% CI 0.19-0.82) and 10 years (HR=6.83, 95% CI 2.29-20.35) (table 2).
Conclusion: The 10-year analysis of this prospective early RA cohort supports a dose and time-dependent impact of very low-dose GC treatment, with a long-term high risk of severe outcomes.
To cite this abstract in AMA style:
Roubille C, Coffy A, Rincheval N, Daures J, Flipo R, Dougados M, Combe B. Ten-year Analysis of the Risk of Severe Outcomes Related to Very Low-dose Glucocorticoids in Early Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/ten-year-analysis-of-the-risk-of-severe-outcomes-related-to-very-low-dose-glucocorticoids-in-early-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ten-year-analysis-of-the-risk-of-severe-outcomes-related-to-very-low-dose-glucocorticoids-in-early-rheumatoid-arthritis/