Session Information
Date: Sunday, October 26, 2025
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The phenotypes of axial spondyloarthritis (axSpA) and clinical responses to placebo and study drugs in randomized controlled trials (RCTs) have changed over time. We conducted a systematic literature review and meta-analysis to evaluate changes in phenotypes and outcomes of axSpA patients participating in RCTs over a 20-year period.
Methods: Systematic search of PubMed, EMBASE, and Cochrane Library, following PRISMA guidelines (PROSPERO registration: CRD42024621406), updated to January 2025. Baseline characteristics were summarized as weighted means or proportions and transformed into standardized mean differences or Log odds ratios for meta-analytic comparisons. To assess temporal trends, categories were based on the first year of recruitment rather than the publication year. Meta-regression was conducted across 5-year intervals (P1:1999-2003, P2:2004-2008, P3:2009-2013, P4:2014-2019). Efficacy outcomes (ASAS20 and ASAS40) were assessed using adjusted models, incorporating comparator group, treatment type, age, BASDAI, radiographic axSpA / Ankylosing Spondylitis, and ASAS classification criteria. Sensitivity analyses used the RoB2 tool.
Results: Ninety RCTs (n=20,411 participants) were included. Descriptive analysis showed that weighted mean ages were lowest during P3 (38.3±5.3 years, 95%CI: 38.1-38.5) and P4 (39.2±4.6 years, 95%CI: 39.2-39.3). Longest disease duration was in P1 (11.6±2.6 years, 95%CI: 11.3-11.6). Male proportion was lowest in P3 (69.2%, 95%CI: 67.8-70.6%) and P4 (71.2%, 95%CI: 70.4-72.0%), and highest HLA-B27 positivity in P4 (79.1%, 95%CI: 78.3-79.8%). Meta-regression showed a significant progressive decrease in male participants (p=0.028, I²=50.2%), A trend towards younger patients was found in recent years, with a significant age decrease in P3 compared with earlier periods (p=0.039). Although meta-analysis revealed a temporal decrease in PtGA scores and an increase in spinal inflammation, no significant temporal trends were detected in meta-regression.Adjusted meta-regressions demonstrated significant efficacy for treatment versus comparator as expected. For ASAS20, efficacy decreased in more recent periods (P3-P4), while no such temporal decrease was observed for ASAS40 (Figure 1A-B). In both adjusted models, bDMARD use as a comparator led to a lower efficacy than placebo as comparator (p< 0.001). Additionally, Higher male proportion was associated with greater efficacy (p< 0.010 in both response criteria).
Conclusion: We report temporal changes in axSpA phenotypes in RCTs over a 20-year period. Progressive reduction in male suggests increased inclusion of women, likely reflecting greater nr-axSpA representation in recent studies. Additionally, a trend toward younger patients was observed, although disease duration remained stable. Despite decreased ASAS20 responses, treatment efficacy remained consistent when assessed by the stricter ASAS40 criteria, particularly after adjusting for key covariates. These findings reflect dynamic changes in recruitment patterns and diagnostic timing, with implications for cross-trial comparability and therapeutic evaluations.
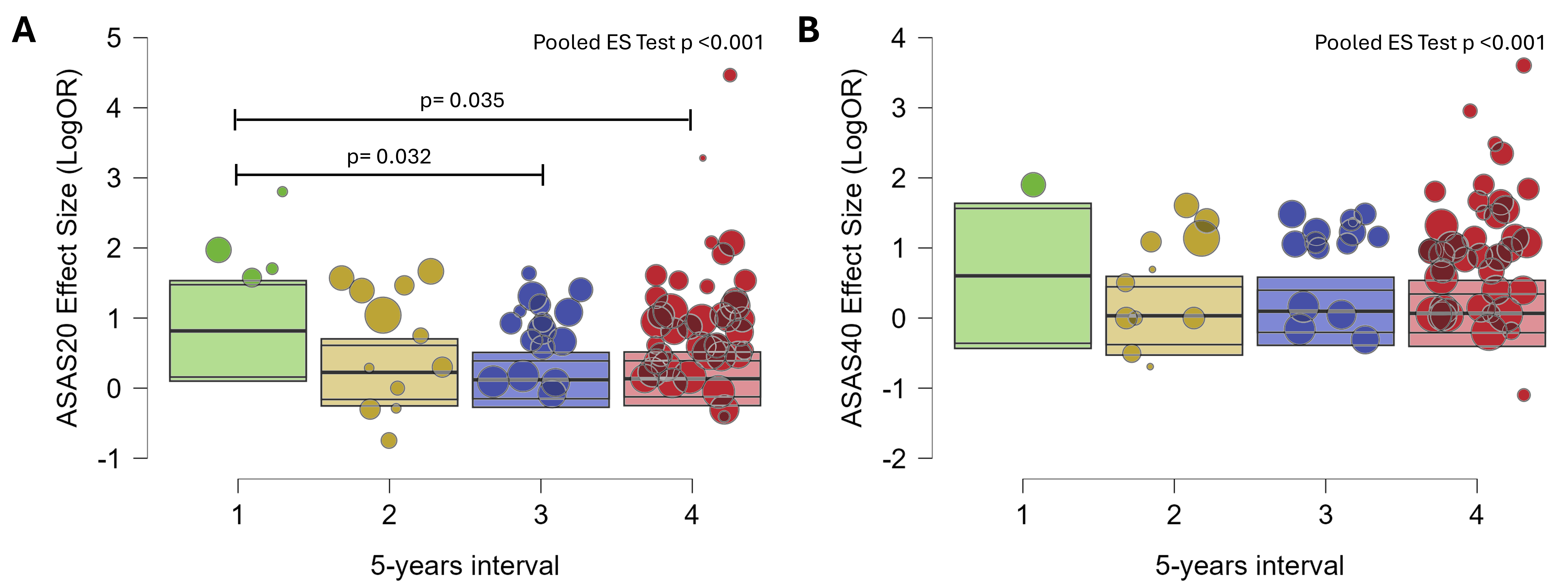 Figure 1. Temporal trends in treatment effect size for ASAS20 and ASAS40 responses across 5-year intervals.
Figure 1. Temporal trends in treatment effect size for ASAS20 and ASAS40 responses across 5-year intervals.
(A) Multivariable-adjusted meta-regression of ASAS20 effect sizes (log odds ratios: LogOR) stratified by 5-year recruitment intervals (P1: 1999–2003; P2: 2004–2008; P3: 2009–2013; P4: 2014–2019). (B) Multivariable-adjusted meta-regression of ASAS40 effect sizes across the same intervals. Each circle represents an individual study, with circle size proportional to study weight in the meta-regression. Boxplots indicate median, interquartile range, and variability of effect sizes within each interval. A significant decline in ASAS20 effect size was observed across periods (p for trend 0.032 and 0.035 for P3 and P4, respectively), whereas ASAS40 effect sizes remained stable over time (p≥0.05).
To cite this abstract in AMA style:
Calixto O, Kiltz U, Lalazi B, Neinert F, Sewerin P, Baraliakos X. Temporal trends in the phenotype and treatment outcomes in axial spondyloarthritis patients included in randomized clinical trials over 25 years: a systematic literature review and meta-regression analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/temporal-trends-in-the-phenotype-and-treatment-outcomes-in-axial-spondyloarthritis-patients-included-in-randomized-clinical-trials-over-25-years-a-systematic-literature-review-and-meta-regression-ana/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/temporal-trends-in-the-phenotype-and-treatment-outcomes-in-axial-spondyloarthritis-patients-included-in-randomized-clinical-trials-over-25-years-a-systematic-literature-review-and-meta-regression-ana/
