Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The role of nonsteroidal anti-inflammatory drugs (NSAIDs) in the management of musculoskeletal symptoms in patients with inflammatory bowel disease (IBD) and IBD-associated arthritis remains controversial, with mixed data on intestinal safety. We aimed to assess real-world use of NSAIDs by capturing temporal trends in prescribing.
Methods: This retrospective cohort study used commercial claims data from 2000-2022 in Optum’s de-identified Clinformatics® Data Mart Database to evaluate adults and children age ≥6 years old meeting validated criteria for a diagnosis of IBD. The proportion of patients with IBD prescribed NSAIDs in each year of the study was evaluated; opioid prescriptions were secondarily assessed. Descriptive statistics were used to assess differences in characteristics between adult and pediatric patients. Wilcoxon-Cruzick test of trend and generalized estimating equation (GEE) logistic regression models were used to evaluate trends in NSAID and opioid prescribing as well as factors associated with NSAID prescription. Linear splines in the GEE models with knots prespecified at 2004 and 2015 were used to capture non-linear temporal trends.
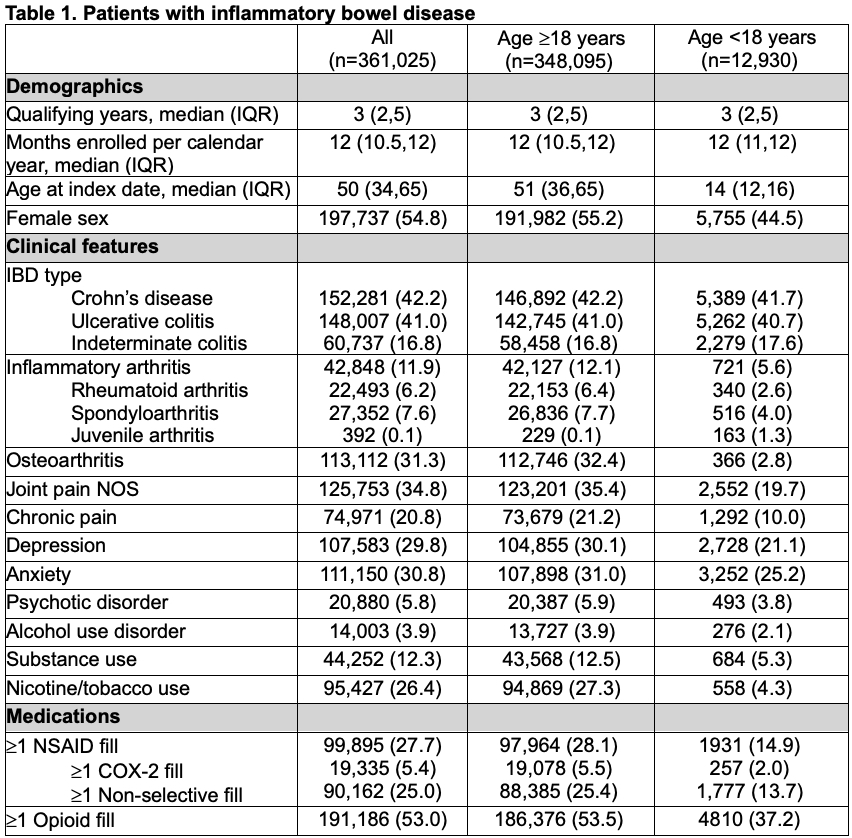
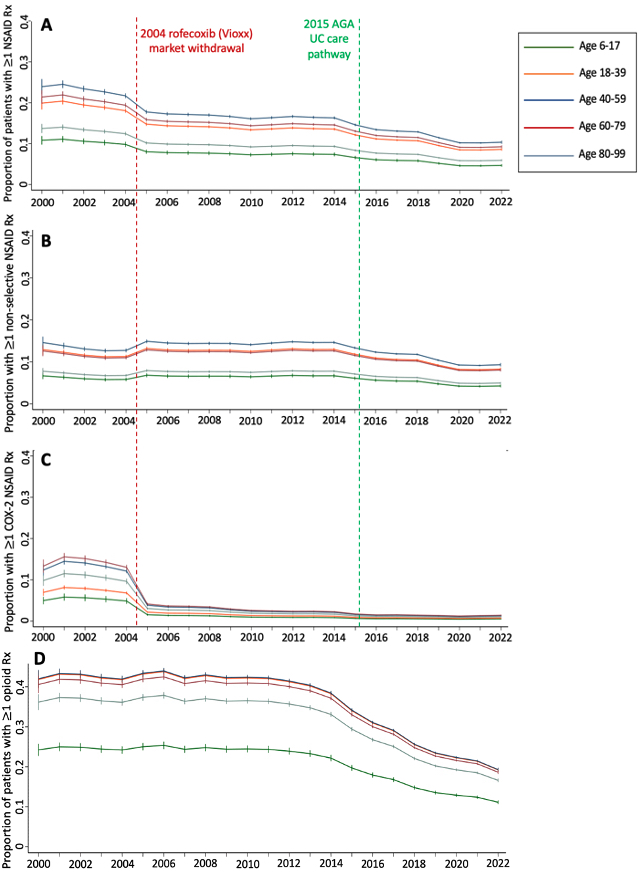
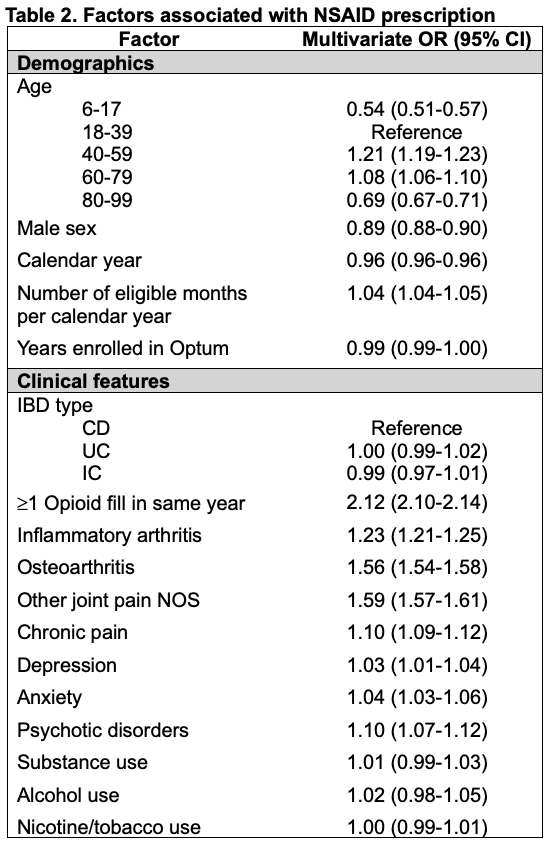
Results: 361,025 patients met inclusion criteria of which 12,930 (3.6%) were < 18 years old. 99,895 (27.7%) patients had at least one prescription NSAID during the study period. Adults were more likely than children to have ≥1 NSAID (28.1% vs. 14.9%, p< 0.01) or opioid prescription (53.5% vs. 37.2%, p< 0.01) (Table 1). Adults were also more likely to have a diagnosis of inflammatory arthritis, osteoarthritis, joint pain not otherwise specified (NOS), or chronic pain (p< 0.01 for all). Prescribing of NSAIDs declined significantly over time (p< 0.01, Figure 1) and was less common than opioid prescription in all calendar years and across all age groups. In the linear spline regression models, there were significant declines in all and COX-2 selective NSAID prescriptions at 2004 and significant declines in all and non-selective NSAID prescriptions at 2015 (p< 0.01 for each). In the multivariable model, opioid prescription (OR 2.12, 95% CI 2.10-2.14), inflammatory arthritis (OR 1.23, 95% CI 1.21-1.25), osteoarthritis (OR 1.56, 95% CI 1.54-1.58), or joint pain NOS (OR 1.59, 95% CI 1.57-1.61) were independently associated with higher odds of NSAID prescription. Conversely, age < 18 (OR 0.54, 95% CI 0.51-0.57) or age ≥80 years (OR 0.69, 95% CI 0.67-0.71), and male sex (OR 0.89, 95% CI 0.88-0.90) were associated with significantly lower odds of NSAID prescription (Table 2).
Conclusion: NSAIDs are used by a sizable minority of patients with IBD but prescription rates have decreased over time and are less common than opioid prescription. Pediatric patients are half as likely to receive NSAIDs compared to adults. Further safety studies are necessary to determine whether NSAIDs should be more frequently considered in patients of all ages with IBD.
Legend: Relevant historical events are indicated by the vertical dashed lines: 1) the Federal Drug Administration withdrawal of the COX_2 selective inhibitor rofecoxib from the United States market in 2004, 2) The 2015 American Gastroenterological Association (AGA) ulcerative colitis (UC) care pathway providing novel national guidance to avoid NSAIDs in patients with IBD. NSAID: non-steroidal anti-inflammatory drug; COX_2: cyclooxygenase_2; Rx: prescription
To cite this abstract in AMA style:
Mayer A, Xiao R, Grossman A, Bewtra M, George M, Weiss P. Temporal Trends in and Associations with NSAID Prescription in Adult and Pediatric Patients with IBD [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/temporal-trends-in-and-associations-with-nsaid-prescription-in-adult-and-pediatric-patients-with-ibd/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/temporal-trends-in-and-associations-with-nsaid-prescription-in-adult-and-pediatric-patients-with-ibd/