Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 7:30AM-9:00AM
Background/Purpose: Telitacicept is a recombinant fusion protein targeting and neutralizing BLyS and APRIL. This phase 3, randomized, double-blind study evaluated the efficacy and safety of telitacicept 160 mg versus placebo in RA patients with an inadequate response to MTX.
Methods: This was a 24-week, randomized, double-blind, placebo-controlled, phase 3 study with an open label treatment follow up period from week 25 to 48 (NCT03016013).Patients with moderate-to-severe RA with an inadequate response to MTX were randomized 3:1 to receive either telitacicept 160 mg or placebo QW for 24 weeks. After Week 24, patients in the placebo arm were switched to telitacicept 160 mg QW for an additional 24 weeks. The primary efficacy endpoint was the proportion of patients achieving an ACR20 response at Week 24.Secondary efficacy endpoints included response rates for ACR50 and ACR70, individual components of the ACR response, DAS28-ESR, and radiographic joint damage as measured by the mTSS at Week 24.
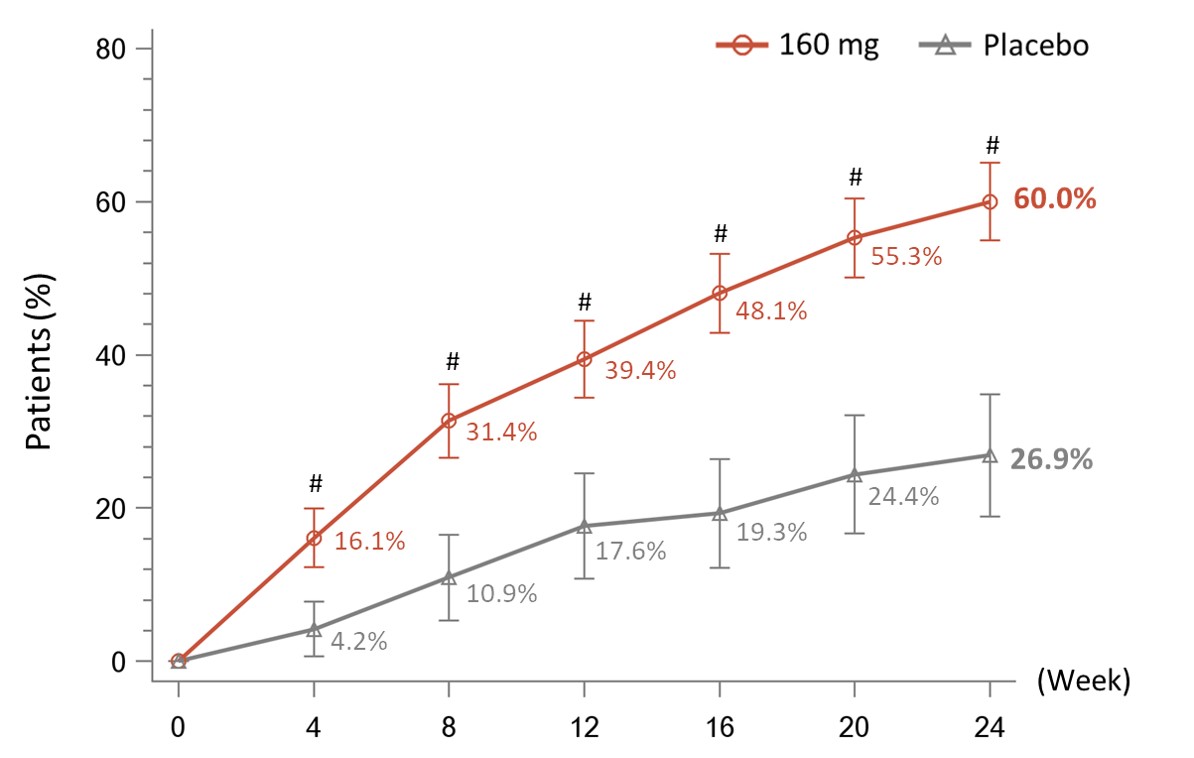
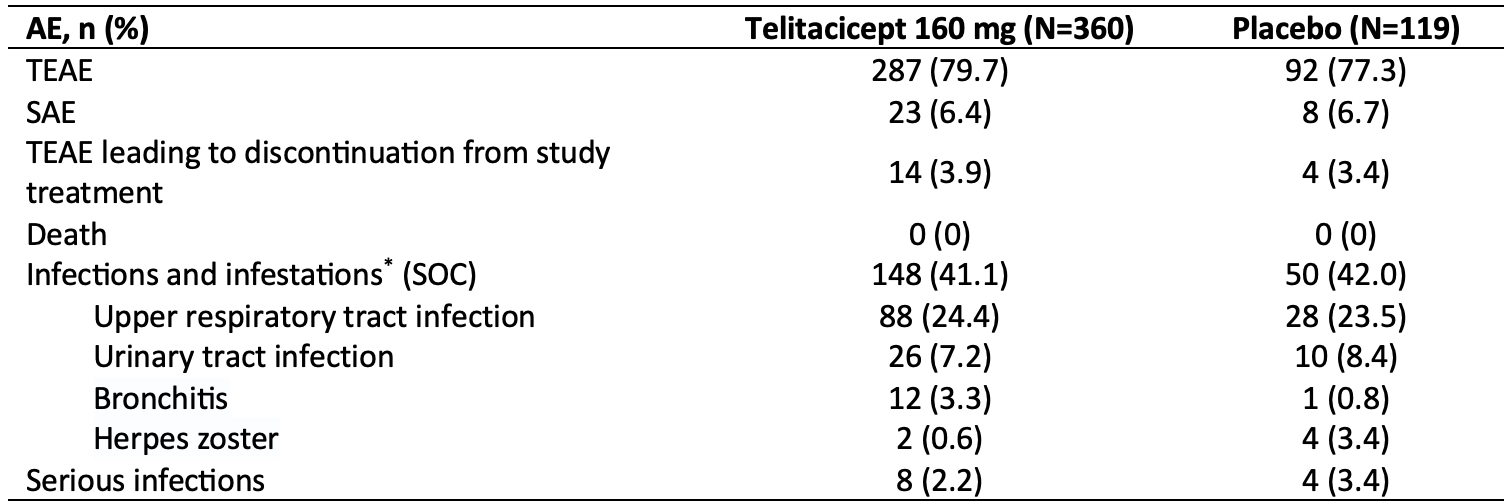
Results: A total of 479 patients were randomized to receive telitacicept 160 mg (N=360) or placebo (N=119). Baseline demographics and disease characteristics were similar between the two groups, with the exception of CRP level which was higher for telitacicept versus placebo (22.856 mg/L vs. 17.287 mg/L).The primary endpoint was met with significantly more patients in the telitacicept 160 mg group achieving an ACR20 response at Week 24 compared with the placebo group (60.0% vs 26.9%, P< 0.001) (Figure 1). At Week 24, the proportion of patients achieving an ACR50 response was significantly higher in the telitacicept 160 mg group compared with placebo (21.4% vs. 5.9%, P< 0.001) and the reductions from baseline in the DAS28-ESR as well as the individual components of the ACR criteria were significantly greater for patients receiving telitacicept compared with placebo (Table 1). Significantly more patients in the telitacicept 160 mg group showed no radiographic progression (△mTSS ≤0) at Week 24 compared with placebo (90.2% vs 66.4%, P< 0.001). Additionally, patients in the telitacicept 160 mg group showed significantly less progression of joint damage (as measured by mTSS, joint space narrowing score, erosion score) from baseline to Week 24 ( Table 1). The incidences of treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), TEAEs leading to discontinuation from study treatment, and infections were similar between the telitacicept 160 mg group and placebo group (Table 2). No patient deaths occurred during the study.
Conclusion: These phase 3 study results demonstrate the efficacy (e.g., responses in the ACR20, ACR50, and DAS28-ESR; and no radiographic progression measured by mTSS) and safety of telitacicept in treating moderate-to-severe RA patients with an inadequate response to MTX.
#P<0.001 vs. Placebo. NRI, missing data were imputed as non-response. FAS, full analysis set.
*Missing data were imputed by last observation carried forward method (LOCF).
+Missing data were not imputed, the observed values were analyzed.
FAS, full analysis set.
AE, adverse event; TEAE, treatment-emergent adverse event; SAE, serious adverse event; SOC, system organ class.
*AEs with an incidence of ≥3% in any group were listed.
To cite this abstract in AMA style:
Wang L, Xu D, Fang J, Zuraw Q, ZHANG F. Telitacicept, a Human Recombinant Fusion Protein Targeting and Neutralizing B Lymphocyte Stimulator (BlyS) and a Proliferation-Inducing Ligand (APRIL), in Rheumatoid Arthritis (RA) Patients with an Inadequate Response to Methotrexate (MTX): A Randomized, Double-Blind, Phase 3 Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/telitacicept-a-human-recombinant-fusion-protein-targeting-and-neutralizing-b-lymphocyte-stimulator-blys-and-a-proliferation-inducing-ligand-april-in-rheumatoid-arthritis-ra-patients-with-an-in/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/telitacicept-a-human-recombinant-fusion-protein-targeting-and-neutralizing-b-lymphocyte-stimulator-blys-and-a-proliferation-inducing-ligand-april-in-rheumatoid-arthritis-ra-patients-with-an-in/