Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: In the wake of the COVID-19 pandemic, telemedicine rapidly became standard of care for people with rheumatic diseases. Observational data on effectiveness and acceptability of telemedicine for rheumatology care is mixed. We aimed to determine whether satisfaction with telemedicine visits was noninferior to in-person visits for delivery of rheumatologic care.
Methods: We conducted a parallel group, randomized, single-blind, noninferiority trial in rheumatology clinics at a tertiary academic medical center. Eligible patients were age ≥ 18 years with one of several rheumatic diseases, and completed ≥ 2 clinic visits in ≤ 18 months (telemedicine or in-person) including ≥ 1 prior visit in-person in ≤ 18 months. The primary outcome was high post-visit satisfaction rate (9 or 10 on a 0 – 10 satisfaction scale). Using an administered survey, we also collected date on preference for the next visit type, self-efficacy for managing medications, and medication adherence. Preference for the next visit type was defined as: same as the group allocation, different than the group allocation or no preference. Success was determined if the satisfaction rate with telemedicine versus in-person visits was non-inferior, using a noninferiority margin of -10%. We performed modified intent-to-treat (mITT), and per protocol (PP) analyses to account for those that crossed over, with age adjustment on the PP population.
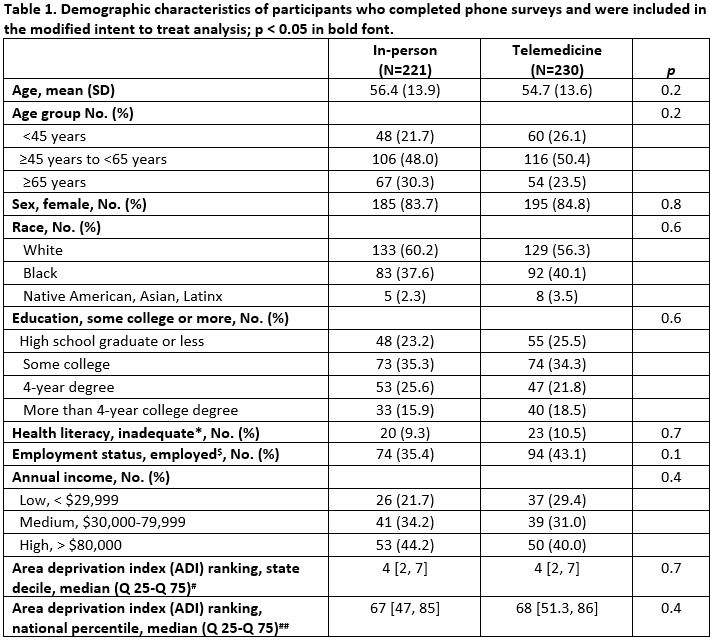
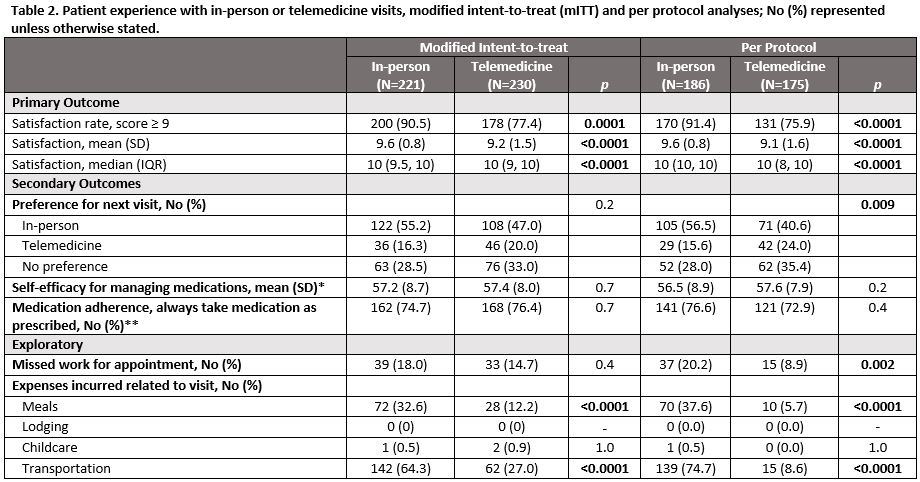
Results: Out of 599 randomized participants, 451, 38.9% Black, 84.3% women, mean age 55.6 completed surveys. In the mITT analysis, telemedicine visits were not non-inferior to in-person visits for the proportion of people that were highly satisfied, 77.4% vs. 90.5%, difference -13.1% (95% CI, -19.8% to -6.5%). 35 participants crossed over in the in-person group and 55 in the telemedicine group. In the PP analysis, the satisfaction rate for telemedicine visits was also not non-inferior to in-person visits, age-adjusted difference -16.2% (95% CI, -23.8% to -8.6%). In the mITT analysis, most participants in both arms preferred an in-person visit for their next visit. The proportion of participants who indicated they preferred the same type of visit for their next visit compared to a different visit type and no preference was significantly greater for those who had in-person visits (55.2% vs 20.0% in-person vs telemedicine, p < 0.0001). There were no differences between groups in self-efficacy for managing medications or medication adherence in either the mITT or PP analyses. Significantly more individuals in the in-person group vs. the telemedicine group reported expenses related to meals and transportation.
Conclusion: Among a large group of diverse, established rheumatology patients, who uniquely were randomized to visit type, high satisfaction rate with rheumatology clinic visits was significantly lower for telemedicine than for in-person visits. A meaningful minority of patients (about 20%) preferred that their next visit be telemedicine. Future studies should focus on factors associated with optimal visit type and how to achieve best outcomes for people with rheumatic diseases, including identifying subgroups that may prefer or benefit most from telemedicine.
Race missing for 1 participant; education missing for 28 individuals; health literacy missing for 16 individuals; income missing for 205 participants; ADI missing for 132 participants.
*PROMIS self-efficacy for managing medication/treatment, reported as the T-score that represents a standardized score with mean of 50 and a standard deviation of 10, and higher scores associated with higher self-efficacy; **from the Medication Adherence Questionnaire.
Self-efficacy for managing medications missing for 15 participants, medication adherence missing for 14 participants.
To cite this abstract in AMA style:
Jackson L, Yazdany J, Aaron K, Goglin S, Margaretten M, Chae D, Curtis J, Paez D, Cutter G, Saag K, Danila M. Telemedicine for Rheumatology Care: A Randomized, Single-Blind, Parallel Group, Noninferiority Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/telemedicine-for-rheumatology-care-a-randomized-single-blind-parallel-group-noninferiority-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/telemedicine-for-rheumatology-care-a-randomized-single-blind-parallel-group-noninferiority-trial/