Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Measurement of proteins from tear fluid represents a non-invasive means to assess the local biology of ocular autoimmune disease such as uveitis. Pediatric uveitis can result from an array of underlying conditions or may be idiopathic. In this study, we leveraged a new proteomic technology, Proximity Extension Assay, to investigate the ocular biology of a diverse cohort of pediatric uveitis patients for classifiers of both disease type and uveitis activity.
Methods: Tear fluid was collected from 50 eyes of 25 patients with pediatric uveitis (active or inactive). Eligible patients were identified by record review from a multidisciplinary uveitis clinic. All patients had a history of anterior uveitis; some also had intermediate or posterior uveitis. Disease activity was based upon pediatric uveitis specialist’s assessment, including: presence of cells; retinitis or use of corticosteroids (CS). Active uveitis was defined as (1) >0.5+ anterior chamber cell and/or ≥1+vitreous cell/haze and/or retinitis or (2) quiet uveitis, along with the use of ≥2 drops/day topical or systemic CS. Tear proteins were eluted from Schirmer’s strips in a standard volume and analyzed using the Olink Explore® platform for 3072 proteins. Only proteins which passed quality control measures were included for analysis. R 4.3.3 was used to perform statistical analysis and data visualizations.
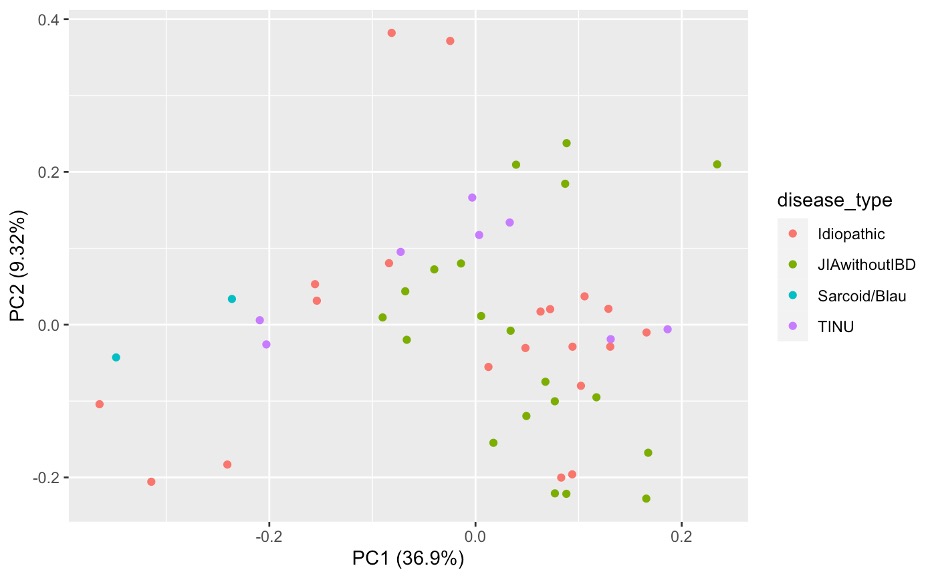
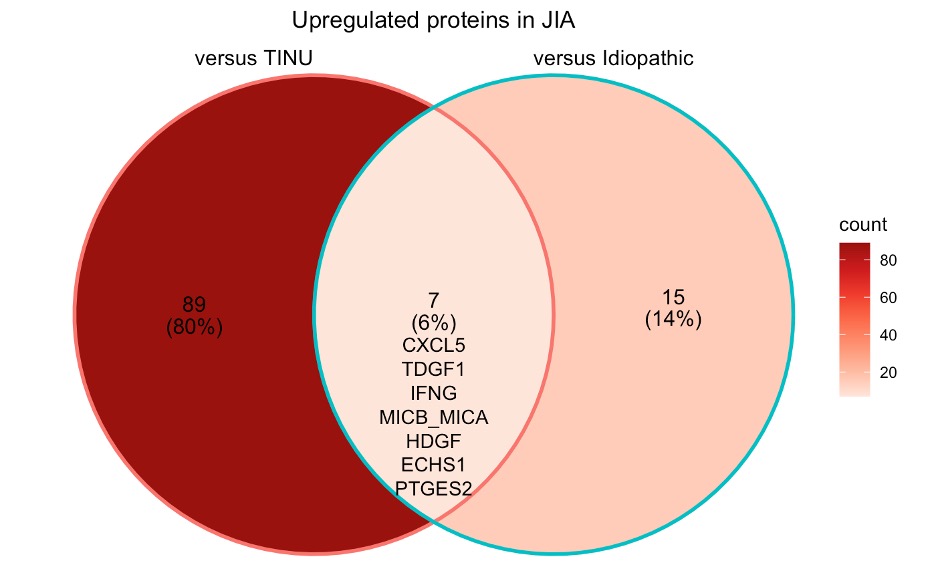
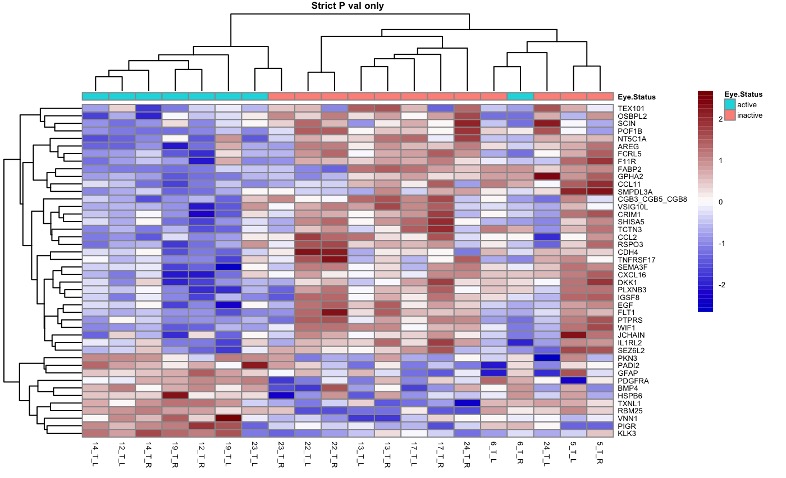
Results: Tears were obtained from patients with JIA-associated uveitis (JIA-U, n=10), Tubulointerstitial nephritis (TINU, n=4), and idiopathic-uveitis (IU, n=10). All had history of anterior uveitis, 2 had Anterior-intermediate uveitis, and 4 pan-uveitis (2 IU, 1 TINU). Eyes clustered in a principal component analysis predominantly by underlying disease (Figure 1). 111 differentially expressed proteins (DEPs) distinguish JIA eyes from TINU and Idiopathic eyes, with 7 unique to JIA as compared to the other diagnoses (Figure 2). Within JIA, unsupervised hierarchical clustering reveals a set of 44 DEPs that robustly classify disease activity (Figure 3). Amongst gene ontology pathways significantly enriched in the DEPs of active eyes, bone morphogenic protein (BMP) signaling represented the highest fold enrichment.
Conclusion: Differential protein expression in easily accessible tear fluid reflects the biology of both underlying disease type and activity state in pediatric uveitis. Because BMP is a member of the anti-inflammatory TGF-b signaling family, future analysis will focus on TGF-b biology as a regulator of inflammatory activity in the eye. Assays of specific protein biomarkers in tear fluid have the potential to become useful diagnostic and prognostic biomarkers in pediatric uveitis.
To cite this abstract in AMA style:
Lerman M, Eckardt D, Davidson S, Behrens E. Tear Proteomics as a Classifier of Disease Type and Activity Status in Pediatric Uveitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/tear-proteomics-as-a-classifier-of-disease-type-and-activity-status-in-pediatric-uveitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tear-proteomics-as-a-classifier-of-disease-type-and-activity-status-in-pediatric-uveitis/