Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
The TaSER study (NCT00920478) is an open label randomized clinical trial with blinded assessments of outcome. It was designed to test whether the efficacy of DAS28 driven treat-to-target DMARD strategies could be improved by the addition of regular musculoskeletal ultrasound (MSUS) disease activity assessment.
Methods:
111 untreated early UA/RA patients (symptom duration <1 year) were randomized to step-up DMARD escalation strategies guided by either DAS28 alone (target = DAS28<3.2) or DAS28+MSUS assessment of a limited joint set (target = PD signal in ≤1 joint). In the MSUS group, ultrasound assessment was undertaken in patients in LDAS, or moderate DAS but with minimal synovitis (28SJC²1). The follow-up period was 18 months. In the first 3 months both groups received identical treatment. Thereafter, treatment was escalated whenever the appropriate target was not achieved; through the addition of new DMARDs, DMARD dose optimisation, and/or IA/IM steroid. The sequence of DMARD escalation was: MTX � MTX/SSZ/HCQ � scMTX/SSZ/HCQ � etanercept/scMTX/SSZ/HCQ.
Outcomes were assessed every 3 months by a metrologist who was blinded to randomization group. The primary clinical outcome was the mean improvement in DAS44. Secondary outcome measures included: mean improvement in ACR core set variables and DAS44 remission rate. Radiological outcomes included the MRI RAMRIS score and Total Sharp Score (results still to be analysed)
Results:
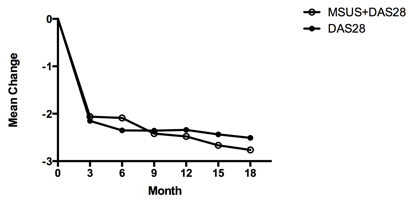
110 (99%) patients fulfilled 2010 ACR/EULAR RA classification criteria. Both groups were well matched for disease duration (median 4m), baseline DAS (4.4), ACPA+ve (60%) and HAQ (1.6 v 1.5). The DAS28 group contained a higher proportion of females (60% vs 78%, p 0.031). Both groups experienced significant improvements in DAS44 (mean change in DAS44 -2.51 [DAS28] vs -2.76 [MSUS]; 95%CI -0.84, 0.33; p 0.39) and HAQ (-0.79 v -1.06; 95%CI -0.57, 0.031: p0.08). The DAS28 group had a numerically higher rate of DAS44 remission after 3 months (43% v 33%; p=0.32). However, after 18 months, more patients in the MSUS groups had attained DAS44 remission (44% v 65%; p=0.046). There was no difference in DAS44, HAQ or ACR core set variables at any time point
Conclusion:
Both groups exhibited similar, very robust improvements in clinical outcomes. MSUS disease activity assessment was not associated with improved clinical outcomes except a higher rate of DAS44 remission after 18 months.
Graph 1 – Mean change in DAS44 from baseline
Graph 2 – Proportion of patients achieving DAS44 remission (* p<0.05)
Disclosure:
J. Dale,
Pfizer Inc,
2;
A. Stirling,
None;
I. B. McInnes,
Pfizer Inc,
2,
Astra Zeneca,
2,
UCB,
5,
BMS,
2,
Pfizer Inc,
5,
Astra Zeneca,
5;
D. Porter,
Pfizer Inc,
2,
Medimmune,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeting-ultrasound-remission-in-early-rheumatoid-arthritis-results-of-the-taser-study/