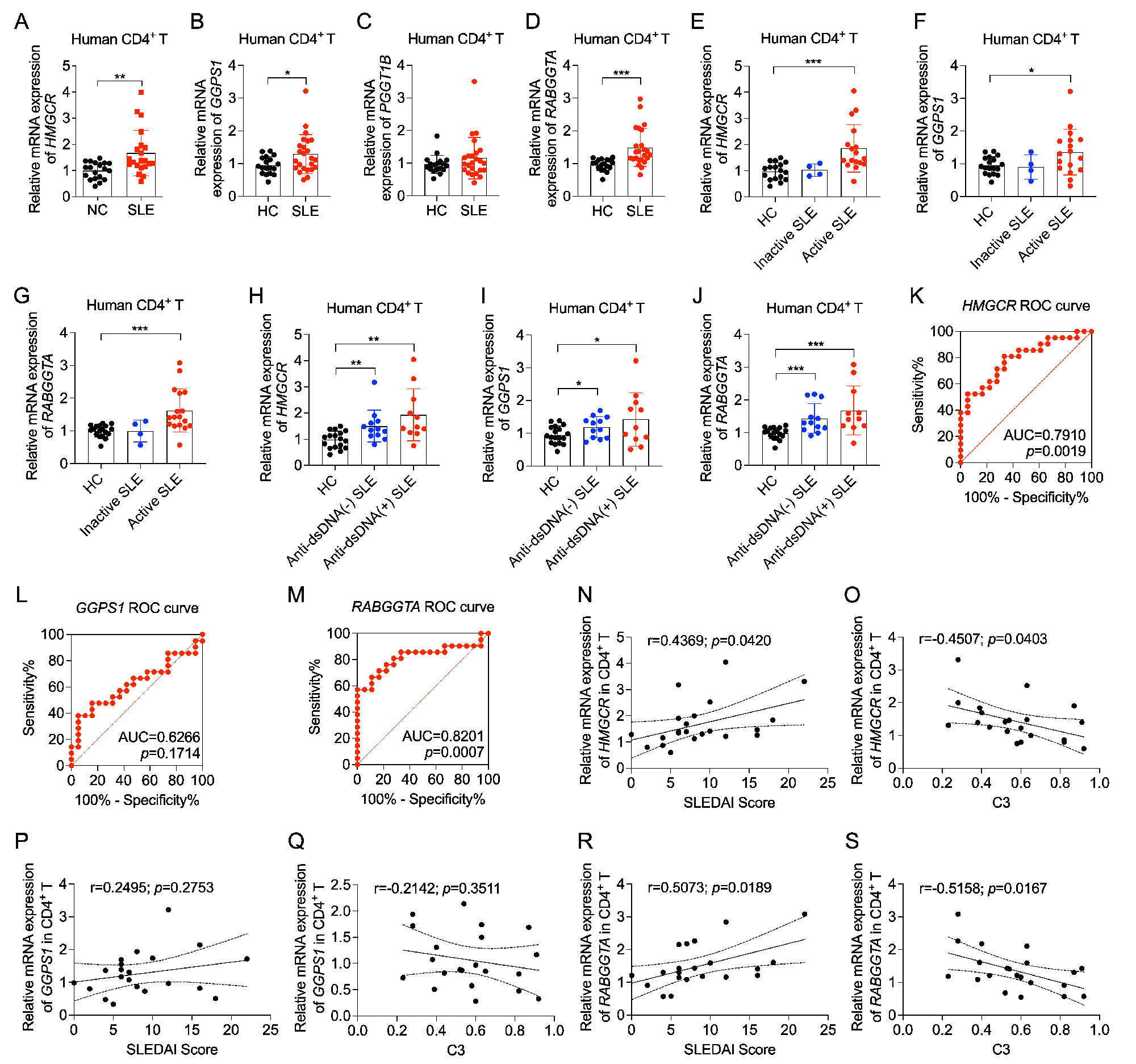Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Recent research focusing on follicular helper T (Tfh) cells emphasizes its importance in autoimmune diseases, such as systemic lupus erythematosus (SLE). However, the mechanisms underlying Tfh cell differentiation remain largely unknown, and therapeutic strategies targeting Tfh cells for SLE are urgently needed.
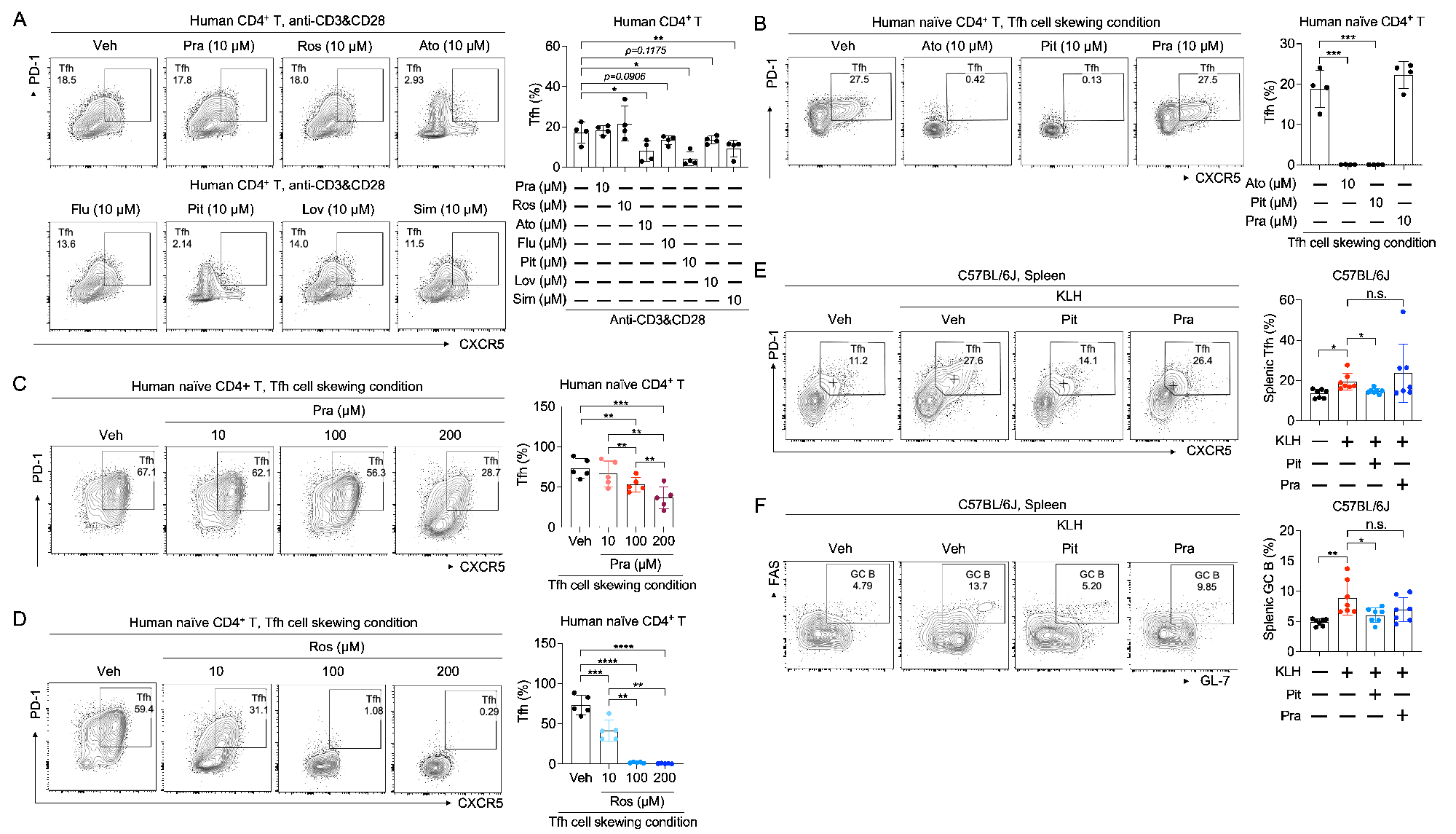
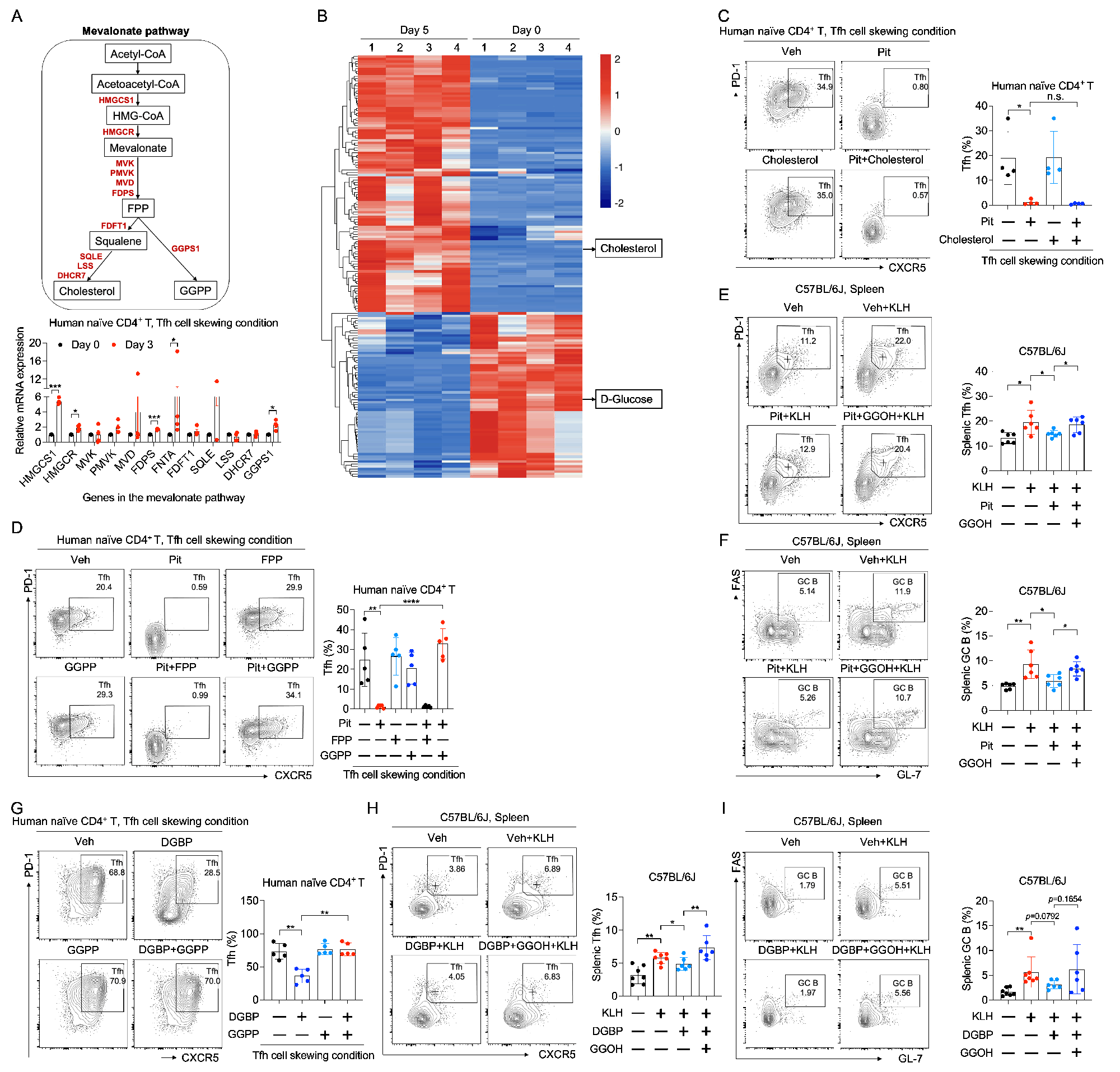
Methods: The effects of seven clinically used statins on human CD4+ T cell subsets were investigated and compared. Chemical and genetic approches were employed to investigate the role of the mevalonate pathway in Tfh cell differentiation and germinal center (GC) reactions in vitro and in vivo. Chemical and genetic approches were subsequently employed to investigate the role of GGPS1 and GTase Ⅱ in Tfh cell differentiation and GC reactions in vitro and in vivo. Expression of the genes in the mevalonate pathway and the genes for protein geranylgeranylation in SLE CD4+ T cells was detected by RT-qPCR and flow cytometry. The role of key genes in the mevalonate pathway and GGTase Ⅱ in CD4+ T cells in SLE pathogenesis was investigated in pristane-induced lupus-like mice. The effects of pitavastatin on SLE were evaluated in lupu prone MRL/lpr mice.
Results: Pharmacological and genetic inhibition of the mevalonate pathway leads to suppressed Tfh cell differentiation and impaired germinal center (GC) reactions. We identify geranylgeranyl pyrophosphate (GGPP) as the key intermediate metabolite derived from the mevalonate pathway driving Tfh cell differentiation. Further studies emphasize that geranylgeranyl transferase Ⅱ (GGTase Ⅱ) facilitates the membrane localization of CXCR5 by regulating the geranylgeranylation of RAB proteins, which transfers the effect of GGPP on Tfh cell differentiation. Conditional ablation of the specific subunit of GGTase Ⅱ, RABGGTA, in CD4+ T cells, leads to impaired Tfh cell differentiation and GC reactions. Moreover, the expression of key genes in the mevalonate pathway and RABGGTA is increased in SLE CD4+ T cells and is positively correlated with SLE disease activity. Genetic ablation of key genes in the mevalonate pathway and RABGGTA postpones the pathological progression in pristane-induced lupus-like mice. Additionally, RAB35 is identified as the key RAB protein that upregulated in SLE CD4+ T cells, and whose expression is positively correlated with SLE disease activity. Point mutation of the Cys sites for geranylgeranylation at the carboxyl terminus of RAB35 impedes the membrane localization of CXCR5 and restricts Tfh cell differentiation both in vitro and in vivo. Finally, lipophilic pitavastatin is effective in ameliorating SLE disease activity.
Conclusion: In summary, our current study highlights the importance of geranylgeranylation of RAB proteins regulated by the mevalonate pathway in Tfh cell differentiation and SLE pathogenesis, and proposes pitvastatin as an adjuvant therapy for SLE.
To cite this abstract in AMA style:
Wang L. Targeting the Mevalonate Pathway-dependent Protein Geranylgeranylation to Restrict Follicular Helper T Cell Differentiation for the Treatment of Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/targeting-the-mevalonate-pathway-dependent-protein-geranylgeranylation-to-restrict-follicular-helper-t-cell-differentiation-for-the-treatment-of-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeting-the-mevalonate-pathway-dependent-protein-geranylgeranylation-to-restrict-follicular-helper-t-cell-differentiation-for-the-treatment-of-systemic-lupus-erythematosus/