Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Many of the inflammatory cytokines implicated in Sjogren’s Disease (SjD) pathogenesis, in particular Type I and II interferons (IFNs), signal through Janus kinases (JAK) – signal transducer and activator of transcription (STAT) pathway. However, a systematic investigation of the role of IFNs and JAK-STAT signaling at the cellular and tissue level in humans is lacking.
Methods: Peripheral blood mononuclear cells (PBMC) and minor salivary gland (MSG) biopsies were obtained from healthy volunteers (HVs) and SjD patients that met the 2016 American College of Rheumatology classification criteria on IRB approved protocols. Research tissues were used for immunofluorescence (IF); RNA sequencing (RNAseq) and single cell RNAseq (scRNAseq) on MSGs. Intracellular phosphorylated STATs (e.g., pSTAT1,3) at baseline and after IFN beta stimulation; with or without JAK inhibitor was assessed in PBMCs using flow cytometry. The effect of IFN beta on the secretory epithelium was modeled using primary salivary gland epithelial cells (pSGEC) from HV (N=3) and SjD patients (N=3) with or without JAK inhibitor using qRT-PCR of ISG gene (CXCL10) and Western immunoblot.
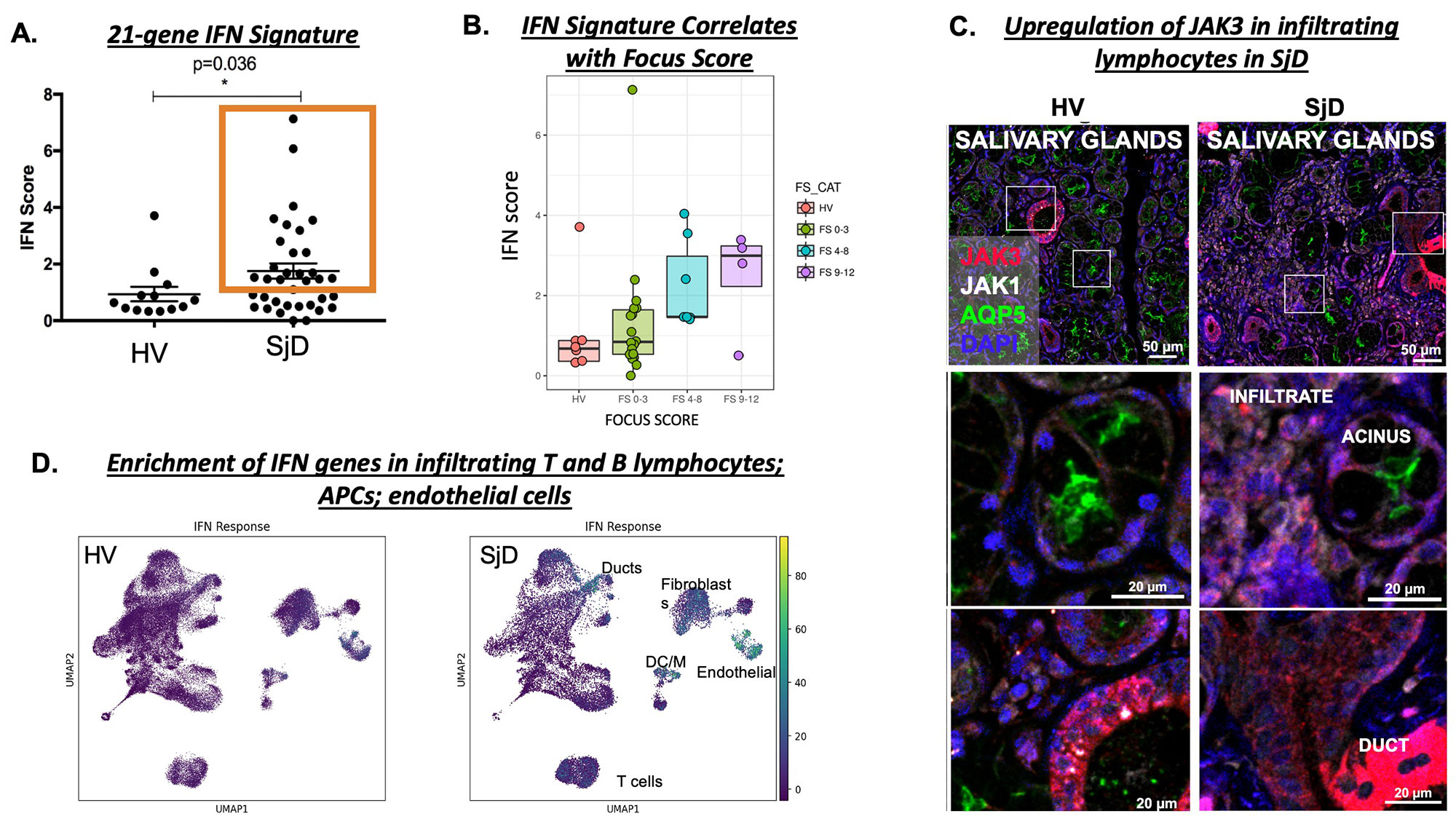
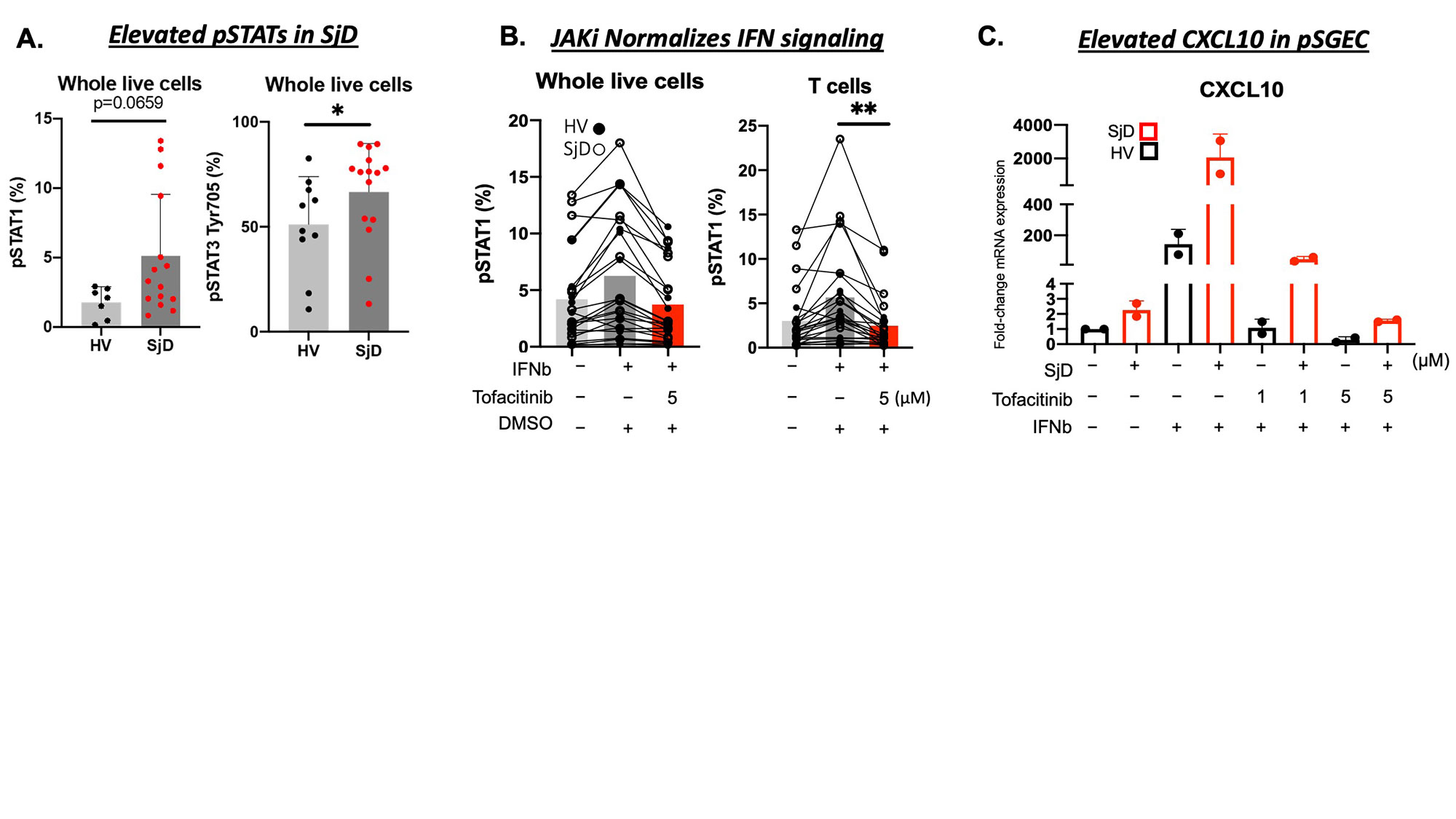
Results: RNAseq on MSGs found enrichment of JAK-STAT pathway genes (e.g., JAK2, JAK3, STAT1) in SjD (n=36) compared to HV (n=13). A composite score of 21-IFN stimulated genes (ISGs) was elevated in SjD patients compared to HVs (Figure 1A) and positively correlated with clinical variables of SjD (e.g., focus scores) (Figure 1B). IF showed increased expression of JAK3 in infiltrating lymphocytes (Figure 1C). To delineate the cells driving this increased IFN signature and highly sensitive to IFN stimulation scRNAseq of MSGs from SjD (n=12) and HVs (n=17) was performed, which showed enrichment of JAK-STAT pathway genes including ISGs in infiltrating T and B lymphocytes; antigen presenting cells; and endothelial cells (Figure 1D). Ex-vivo studies showed baseline elevation of intracellular pSTAT1 and pSTAT3 levels in PBMCs from patients with SjD (n=15) compared to HVs (n=10) (Figure 2A). Stimulation with IFN beta further increased the pSTAT1 signaling and tofacitinib-mediated JAK inhibition led to normalization of JAK-STAT signaling (Figure 2B). HV and SjD-derived pSGEC showed elevated basal CXCL10 in patients with SjD (n=3) vs HV (n=3); and tofacitinib was able to block IFN beta driven ISG expression (Figure 2C).
Conclusion: Interferon signaling through the JAK-STAT pathway is activated in the MSGs from patients with SjD. In this study, T cells are the most significantly enriched cell type, and endothelial cells along with infiltrating T and B lymphocytes appear to be exquisitely responsive to IFN stimulation. JAK-STAT signaling is activated at baseline in peripheral blood and in the salivary glands and can be normalized by use of JAK inhibition (e.g., tofacitinib). Our findings pinpoint cells responsive to JAK inhibition and illustrate in patient tissues that JAK inhibitors may be beneficial in SjD by uncoupling the pathogenic cytokine milieu and resultant epithelial tissue damage and dysfunction central to SjD. These data can serve as biological endpoints for clinical trials [NCT04496960].
To cite this abstract in AMA style:
Gupta S, Yamada E, Nakamura H, Khavandgar Z, Martin D, Tandon M, Alevizos I, Jang S, Perez P, Dominick K, Pranzatelli T, Baer A, chiorini j, Warner B. Targeting Janus Kinase Pathway in Sjogren’s Disease Corrects IFN-Driven Inflammation and Epithelial Dysfunction [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/targeting-janus-kinase-pathway-in-sjogrens-disease-corrects-ifn-driven-inflammation-and-epithelial-dysfunction/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeting-janus-kinase-pathway-in-sjogrens-disease-corrects-ifn-driven-inflammation-and-epithelial-dysfunction/