Session Information
Session Type: Abstract Session
Session Time: 2:30PM-2:45PM
Background/Purpose: Sjӧgren’s syndrome (SS) is a systemic autoimmune disease that is associated with a lymphoma risk 14-fold that of the general population. Greater focal lymphocytic infiltrate of salivary glands (SGs) and high SG IFNg are associated with elevated lymphoma risk. Interestingly, IFNg is also biologically relevant to mesenchymal stromal cells (MSCs), a SG resident cell with unique regenerative and immunoregulatory capacity. In contrast to the role of IFNg in SS, IFNg is considered to promote a beneficial MSC immunomodulatory phenotype. The objective of this study is to define the immunobiology of IFNg-exposed SG MSCs with and without the JAK inhibitor ruxolitinib.
Methods: All SS subjects fulfilled ACR/EULAR criteria. Sicca-control subjects had symptoms of dryness but did not have diagnoses of autoimmune disease or laboratory abnormalities supportive of an autoimmune disease. SG MSCs were isolated from minor salivary gland tissue and frozen for storage. SS SG MSCs were treated with IFNg 10 ng/mL +/- various doses of ruxolitinib. Experimental methods included flow cytometry, RNA-Sequencing, chemokine array, ELISA, and transwell experiments.
Results: We found that the IFNg promoted expression of MSC immunomodulatory surface markers, and this expression was reversed by 1 µM ruxolitinib (Figure 1A-C). Accordingly, we performed RNA-Sequencing on MSCs pretreated with IFNg +/- 1 µM ruxolitinib. RNA-Sequencing of SS and control SG MSCs showed nearly identical responses IFNg inhibition with ruxolitinib (Figure 1D & E). Because several chemokines were highlighted in the RNA-Sequencing results, we performed a chemokine array on conditioned media from SG-MSCs treated with IFNg +/- ruxolitnib and identified candidate chemokines for further investigation (Figure 2A & B). We confirmed the differential expression of CXCL9, CXCL10, CXCL11, CCL2, and CCL7 using ELISA (Figure 2C). We then sought to define the effect of IFNg and ruxolitinib on MSC-induced PBMC migration (Figure 3A). MSCs promote migration alone, but PBMC migration is amplified by pre-treatment of SG-MSCs with IFNg (Figure 3B). Ruxolitinib reverses this increased migration, though not reaching statistical significance (Figure 3C). We determined CD4+ T cells migrate significantly more with IFNg (p=0.04) and that neutralizing CXCL9, CXCL10, and CXCL11 attenuated IFNg-induced migration (Figure 3D).
Conclusion: These findings establish that ruxolitinib mitigates IFNg-induced expression of immunomodulatory surface markers and chemokines expressed by SG-MSC. Ruxolitinib also reverses IFNg-induced PBMC migration, likely through acting on CXCL9, 10, and 11 to reduce CD4+ T cell migration. Because IFNg is higher in SS than control SGs, we have identified SG MSCs as a plausible pathogenic cell type in SS, promoting CD4+ T cell chemotaxis within SS SGs. Further, we have shown ruxolitinib reverses the effect of IFNg on key chemokine expression, providing a possible therapeutic avenue. Future studies will confirm our findings with ex vivo and mouse model studies
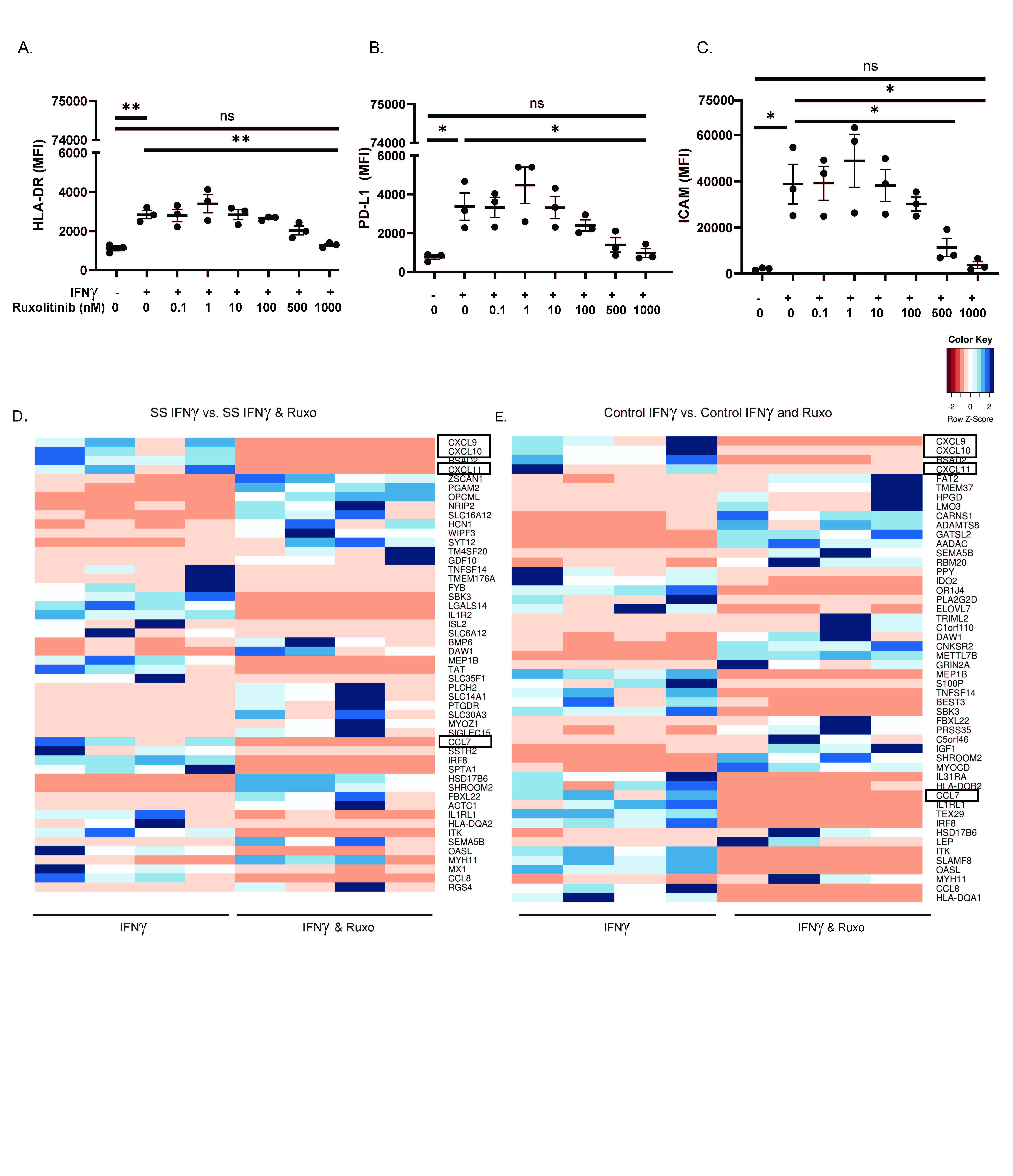 Figure 1. Ruxolitinib reverse IFNγ-induced SG-MSC immunomodulatory protein expression and transcript profile. SG-MSCs were treated +/- IFNγ for 48 hours +/- ruxolitinib. A-C) Median immunofluorescence intensity (MFI) of MSC immunomodulatory markers HLA-DR, PD-L1, and ICAM (n=3); D-E) Transcriptomic profiling of SS (n=4) and control (n=4) SG-MSCs. Heat maps display the top differentially regulated genes filtered for an adjusted p value of < 0.05. Boxes highlight differentially expressed chemokines. .ns=not significant; *=p < .05; **=p < 0.01
Figure 1. Ruxolitinib reverse IFNγ-induced SG-MSC immunomodulatory protein expression and transcript profile. SG-MSCs were treated +/- IFNγ for 48 hours +/- ruxolitinib. A-C) Median immunofluorescence intensity (MFI) of MSC immunomodulatory markers HLA-DR, PD-L1, and ICAM (n=3); D-E) Transcriptomic profiling of SS (n=4) and control (n=4) SG-MSCs. Heat maps display the top differentially regulated genes filtered for an adjusted p value of < 0.05. Boxes highlight differentially expressed chemokines. .ns=not significant; *=p < .05; **=p < 0.01
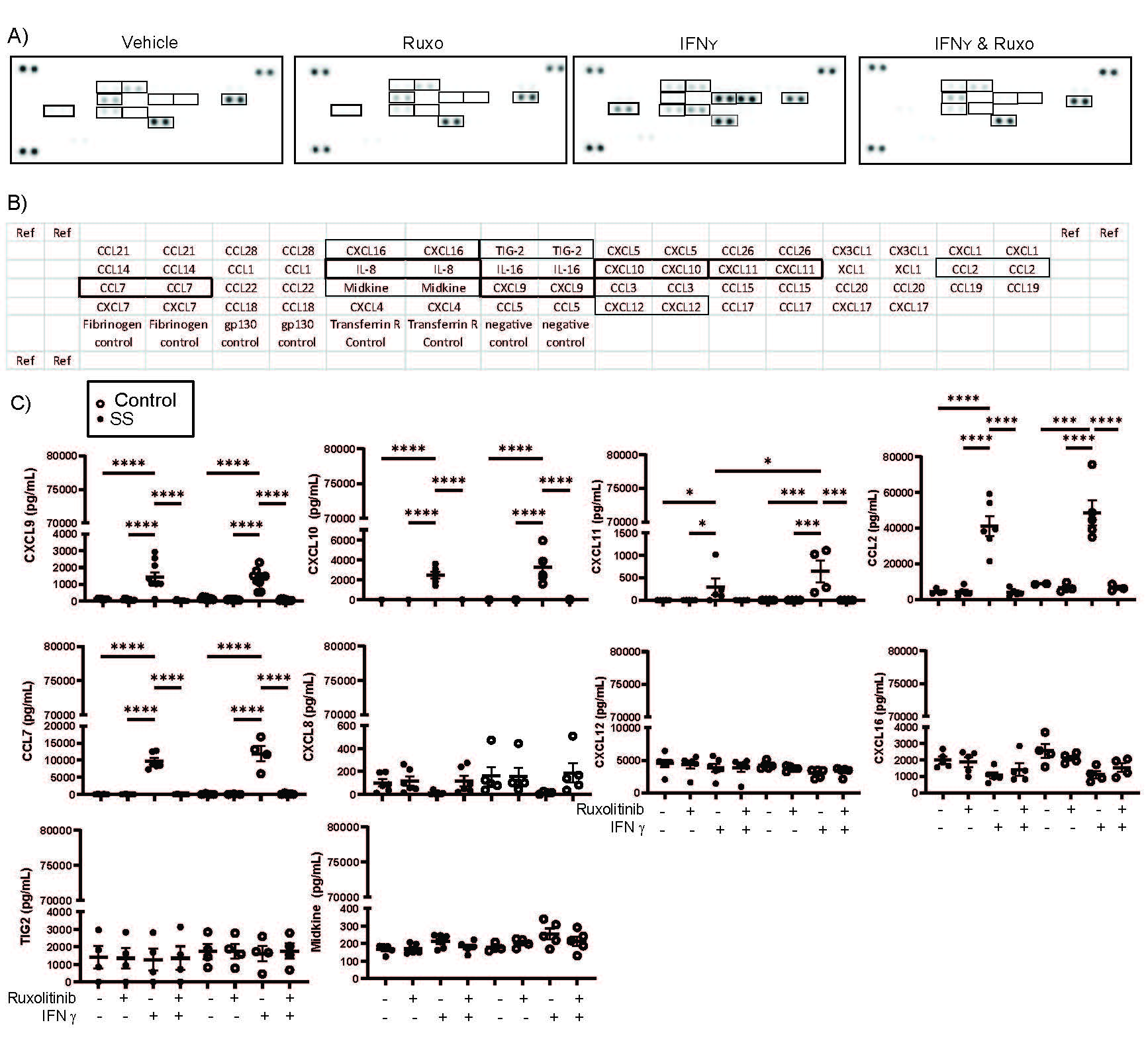 Figure 2. IFNγ-induced production of CXCL9, CXCL10, CXCL11, CCL2, & CCL7 in salivary gland MSCs is reversed by ruxolitinib. Salivary gland MSCs were culturedwith IFNγ +/- 1000 nM ruxolitinib or vehicle for 48 hours. After 48 hours, conditioned media was collected for further study. Conditioned media was applied to a chemokine array per the manufacturer’s specifications. Quantitative analysis using densitometry was performed. ELISA was performed on the conditioned media for each chemokine identified as differentially expressed in the chemokine array per manufacturer’s recommendations. A) Images of the chemokine membrane incubated with conditioned media derived from vehicle treated, IFNγ treated, ruxolitinib treated, or both IFNγ and ruxolitinib-treated salivary gland MSCs; B) Key identifying the layout of the chemokine array; C) Confirmation of the chemokine array was performed using the conditioned media ELISA including CXCL9, CXCL10, CXCL11, CCL2, CCL7, CXCL8, CXCL12, CXCL16, TIG2, and midkine. ELISAs were performed on control (n=5) and SS (n=6) subjects.*=p < 0.05; **=p < 0.01; ***=p < 0.001; ****=p < 0.0001
Figure 2. IFNγ-induced production of CXCL9, CXCL10, CXCL11, CCL2, & CCL7 in salivary gland MSCs is reversed by ruxolitinib. Salivary gland MSCs were culturedwith IFNγ +/- 1000 nM ruxolitinib or vehicle for 48 hours. After 48 hours, conditioned media was collected for further study. Conditioned media was applied to a chemokine array per the manufacturer’s specifications. Quantitative analysis using densitometry was performed. ELISA was performed on the conditioned media for each chemokine identified as differentially expressed in the chemokine array per manufacturer’s recommendations. A) Images of the chemokine membrane incubated with conditioned media derived from vehicle treated, IFNγ treated, ruxolitinib treated, or both IFNγ and ruxolitinib-treated salivary gland MSCs; B) Key identifying the layout of the chemokine array; C) Confirmation of the chemokine array was performed using the conditioned media ELISA including CXCL9, CXCL10, CXCL11, CCL2, CCL7, CXCL8, CXCL12, CXCL16, TIG2, and midkine. ELISAs were performed on control (n=5) and SS (n=6) subjects.*=p < 0.05; **=p < 0.01; ***=p < 0.001; ****=p < 0.0001
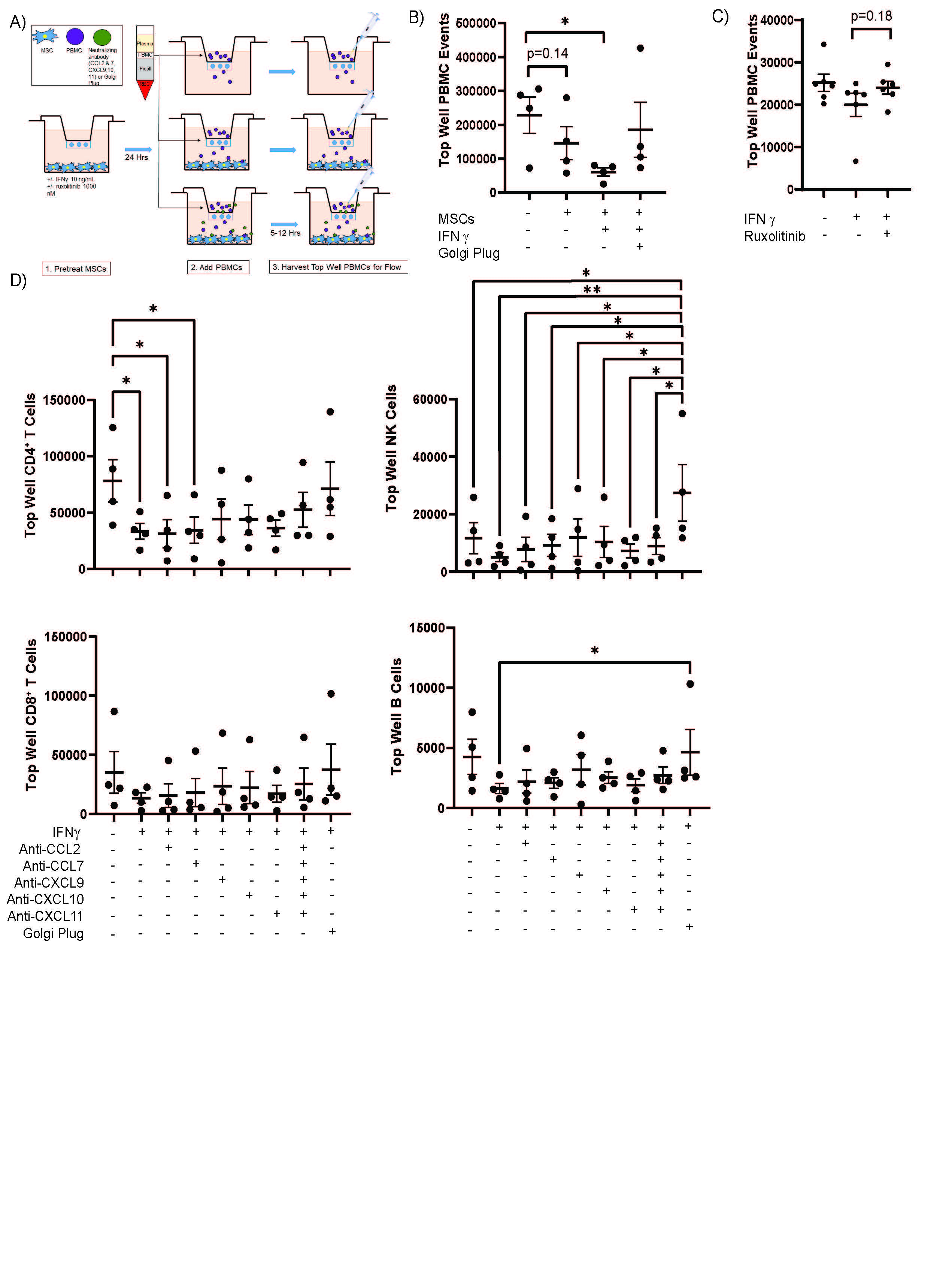 Figure 3. MSCs Promote PBMC Migration, an effect amplified by IFNγ. MSCs were plated onto the bottom of a transwell. MSCs were serum starved for 24 hours then treated +/- IFNγ or ruxolitinib for another 24 hours. The MSCs were washed well then PBMCs were added into the top well. After 5_12 hours, the cells remaining in the top well were collected, stained, and subjected to flow cytometry. A) Representative figure of the transwell experimental setup; B) MSCs (n=1)were treated with IFNγ 10 ng/mLfor 24 hours, washed, then PBMCs (n=4) were added into the top of a five micron transwell system for five hours. IFNγ increased PBMC migration across the transwell filter. C) MSCs (n=6) were treated with IFNγ 10 ng/mL +/- or 1000 nM ruxolitib for 24 hours, washed, then PBMCs (n=1) were added into the top of an eight micron transwell system for five hours. Ruxolitinib abrogates the pro-migratory effect of IFNγ. D) MSCs (n=1) were pre-treated with IFNγ then PBMCs (n=4) were added to the top well of a 5 micron transwell system in the presence of neutralizing antibodies or golgi plug. Data were presented as the number of lymphocytes from the top well (cells that did not migrate). CD4+ T cells migrated most robustly in the presence of IFNγ and neutralizing antibodies to CXCL9, 10, and 11 reduced migration. A similar less significant trend was seen in CD8+ T cells, B cells, and NK cells. *=p < 0.05; **=p < 0.01,
Figure 3. MSCs Promote PBMC Migration, an effect amplified by IFNγ. MSCs were plated onto the bottom of a transwell. MSCs were serum starved for 24 hours then treated +/- IFNγ or ruxolitinib for another 24 hours. The MSCs were washed well then PBMCs were added into the top well. After 5_12 hours, the cells remaining in the top well were collected, stained, and subjected to flow cytometry. A) Representative figure of the transwell experimental setup; B) MSCs (n=1)were treated with IFNγ 10 ng/mLfor 24 hours, washed, then PBMCs (n=4) were added into the top of a five micron transwell system for five hours. IFNγ increased PBMC migration across the transwell filter. C) MSCs (n=6) were treated with IFNγ 10 ng/mL +/- or 1000 nM ruxolitib for 24 hours, washed, then PBMCs (n=1) were added into the top of an eight micron transwell system for five hours. Ruxolitinib abrogates the pro-migratory effect of IFNγ. D) MSCs (n=1) were pre-treated with IFNγ then PBMCs (n=4) were added to the top well of a 5 micron transwell system in the presence of neutralizing antibodies or golgi plug. Data were presented as the number of lymphocytes from the top well (cells that did not migrate). CD4+ T cells migrated most robustly in the presence of IFNγ and neutralizing antibodies to CXCL9, 10, and 11 reduced migration. A similar less significant trend was seen in CD8+ T cells, B cells, and NK cells. *=p < 0.05; **=p < 0.01,
To cite this abstract in AMA style:
McCoy S, Gurevic I, Parker M, Galipeau J. Targeting Endogenous Mesenchymal Stromal Cell Response to Interferong in Sjӧgren’s Syndrome [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/targeting-endogenous-mesenchymal-stromal-cell-response-to-interferong-in-sj%d3%a7grens-syndrome/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeting-endogenous-mesenchymal-stromal-cell-response-to-interferong-in-sj%d3%a7grens-syndrome/
