Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: In about 10% of patients, the immune response to immune checkpoint inhibitors (ICI) exceeds the anti-tumor response and leads to autoimmune complications (immune-related Adverse Events, irAEs), which can sometimes be severe and require the use of targeted therapies. Two studies recently reported a deleterious impact of targeted therapies on the survival of patients with irAEs. This work aims at comparing overall survival in patients treated for an hospitalized irAE with a biological or targeted synthetic DMARD (b/tsDMARD), conventional synthetic DMARD or exclusively with corticosteroids.
Methods: All adults included in the French national health database who initiated ICI for any type of cancer between 2014 and 2019 were retrospectively analyzed, with a a one-year look-back and one year look-ahead period. The occurrence of an irAE during ICI treatment or in the 12 following months was defined by the combination of (1) hospitalization for a cause evoking an irAE of any nature, except endocrinopathy, and (2) initiation of corticosteroids, csDMARD or b/tsDMARD. Patients contributed from the time the 2 criteria were fulfilled until the end of follow-up or death. Treatment of IRAEs was considered as a time dependent variable with 3 classes: corticosteroids alone, csDMARD and b/tsDMARD. Because switches in treatment could occur during follow-up, propensity score (PS) was recalculated every 30 days using a multinomial logistic regression. PS included the current treatment, gender, age, type of cancer, ICI prescribed in monotherapy or as a combination, ICI year of beginning, time from cancer to initiation of ICI, use of corticosteroids, hospitalization in intensive care unit, type of irAE, number of LTDs, Charlson’s index and FDep social deprivation index. Overall survival was compared between the three groups using a Cox model ponderated with overlap weights to ensure balance.
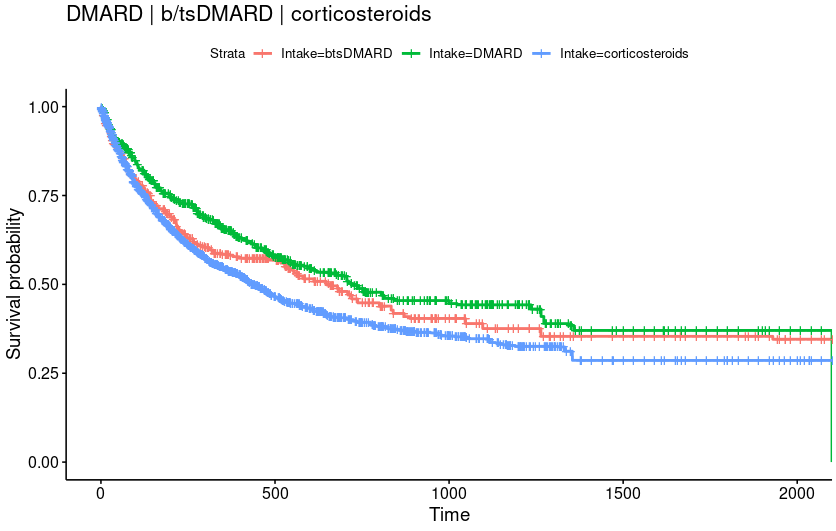
Results: 71,723 patients (men: 66.0%, median age: 66 years) initiating an ICI were analyzed. An hospitalized irAE occurred in 2575 patients at a median time of 232 days after ICI initiation and the 4 most frequent were gastrointestinal (inflammatory colitis, 1.7%), rheumatic (0.7%), cardiologic (0.5%) and pulmonary (0.4%). Global mortality was 66.0%. Median patient follow-up after irAE was 268 days. The weighted baseline populations consisted of 180 patients treated with b/tsDMARD, 263 patients treated with conventional DMARD and 341 patients exclusively with corticosteroids. Covariates were well-balanced between groups.
Overall mortality in patients treated with b/tsDMARD did not significantly differ from csDMARD (HR=1.13, CI=[0.97, 1.33]). Overall mortality in patients treated with corticosteroids was significantly higher than csDMARD (HR=1.30, CI=[1.04, 1.64]) but did not differ significantly from b/tsDMARD (HR=1.15, CI=[0.95, 1.39]).
Conclusion: In this large nationwide study, with one of the largest number of hospitalized irAEs and b/tsDMARD-treated patients, targeted therapies were not associated with a worse prognosis. The prognosis according to the underlying type of IRAEs is currently being investigated and will be presented at the meeting.
To cite this abstract in AMA style:
Gottenberg J, Sedmak N, Tran Ba Loc P, Fabacher T, Bahougne T, Arnold C, Desjeux G, Servy H, Meyer N, Sauleau E, Seror R, Sebbag E. Targeted Therapies for Severe Immune-related Adverse Events of Immune Checkpoint Inhibitors Are Not Associated with a Worse Prognosis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/targeted-therapies-for-severe-immune-related-adverse-events-of-immune-checkpoint-inhibitors-are-not-associated-with-a-worse-prognosis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeted-therapies-for-severe-immune-related-adverse-events-of-immune-checkpoint-inhibitors-are-not-associated-with-a-worse-prognosis/