Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Upadacitinib (UPA), an oral JAK inhibitor selective for JAK1, demonstrated efficacy in patients with active ankylosing spondylitis (AS) with an inadequate response (IR) to nonsteroidal anti-inflammatory drugs (NSAID) therapy in the SELECT-AXIS 11 trial.
To identify pathways modulated by UPA in AS patients with emphasis on those associated with response to treatment.
Methods: A subgroup of patients from the SELECT-AXIS 1 study, with available baseline and at least one follow up plasma sample during the placebo-controlled period, were selected for analysis (PBO, n= 65; UPA 15 mg QD, n=63). The levels of 92 inflammation related protein biomarkers (BioMs) were analyzed using the Olink® platform; change from baseline were expressed as Log2 Fold Change; a repeated measure mixed linear model identified BioMs differentially modulated by UPA. Relationship between change in BioMs and change in clinical disease activity measures were derived using Pearson’s correlation (ASDAS-CRP, BASDAI, and CRP) and Spearman’s correlation (MRI Spine SPARCC ). Pathway analysis was performed with Ingenuity® Pathway Analysis (Qiagen Inc.).
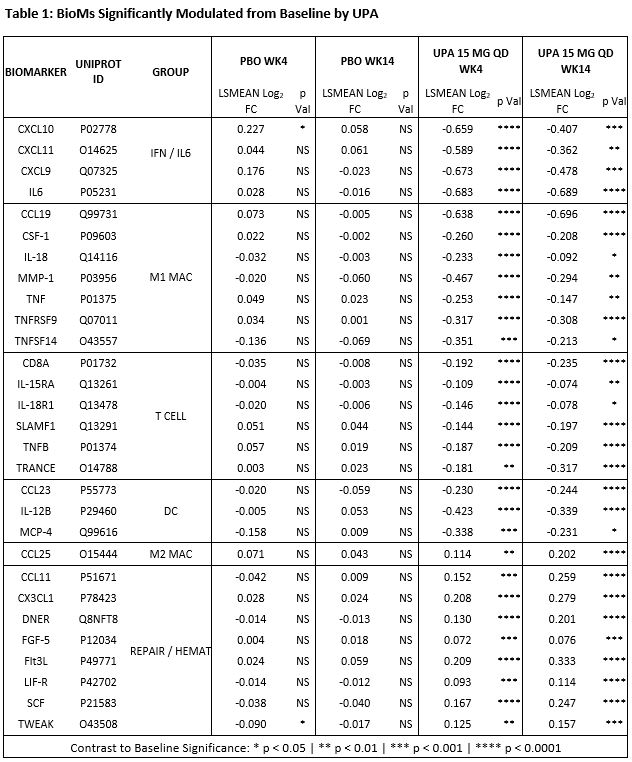
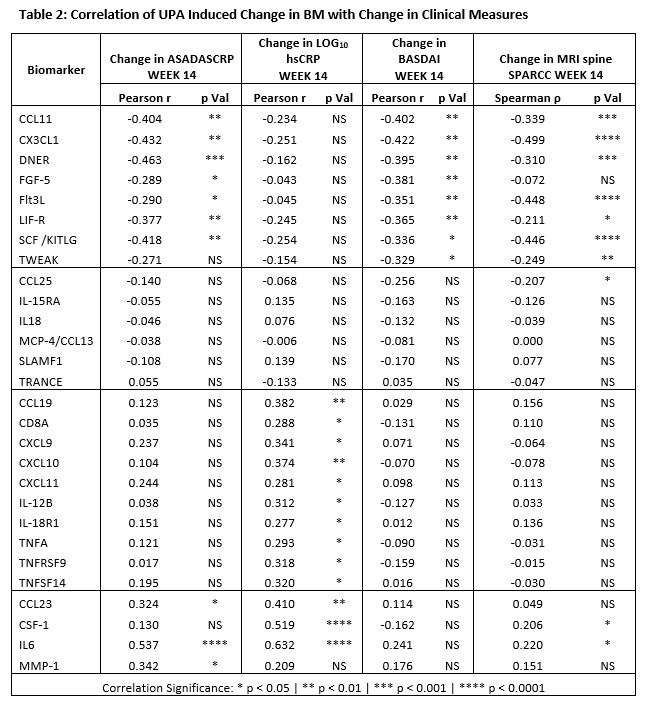
Results: Treatment with UPA 15 mg QD reduced the levels of BioMs associated with IFN, IL6, T Cells, M1 or “inflammatory” Macrophages, and Dendritic Cells (DC); and increased those of BioMs associated with tissue repair and hematopoiesis (Table 1). The type of pathways, inferred in silico based on BM data, suggests that UPA exerts broad inhibitory activity directly on multiple JAK1-dependent (IFNα/β, IFNγ, IL6, IL2, IL5, IL7, and OSM) and indirectly on JAK1-independent upstream pathways (IL1, IL23, IL17, IL18, and TNFα), resulting in the inhibition of key functional pathways such as leukocyte activation and mobility, inflammatory response, and damage to connective tissue. Improvement in ASDAS-CRP, BASDAI, and MRI spine SPARCC correlated with increase in BioMs associated with tissue repair (FGF5, DNER [Delta/Notch Like EGF Repeat Containing]) and hematopoiesis (FLT3LG,and SCF/KITLG), while improvement in ASDAS-CRP and CRP correlated with decrease in CCL23, CSF1, IL-6, and MMP1; and reduction in only CRP correlated with decrease in IFN‑ and of TNFα-related BioMs (Table 2).
Conclusion: Treatment of NSAID-IR AS patients with UPA 15 mg QD resulted in the coordinated decrease in multiple BioMs associated with the innate and adaptive immune responses, and in the increase in BioMs generally associated with tissue repair and hematopoiesis. In silico pathway prediction indicates that treatment with UPA directly inhibits JAK1-dependent and indirectly JAK1-independent pathways, resulting in the down modulation of functional pathways related to inflammation and tissue damage which are known to be dysregulated in AS2. Based on this observation and on the correlation of change in BioMs with change in clinical measures, we hypothesize that both increase in BioMs associated with tissue repair and hematopoiesis, and decrease in BioMs associated with inflammation may contribute to the clinical activity of UPA in AS patients.
References:
1. van der Heijde, D. et al. Lancet 394, 2108-2117 (2019).
2. Stoll, M.L. Clin Exp Rheumatol 29, 322-330 (2011).
To cite this abstract in AMA style:
Sornasse T, Song I, Radstake T, McGonagle D. Targeted Serum Proteomic Analysis Following Upadacitinib Treatment in Ankylosing Spondylitis Shows Robust Suppression of Innate and Adaptive Immune Pathways with Tissue Repair Modulation [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/targeted-serum-proteomic-analysis-following-upadacitinib-treatment-in-ankylosing-spondylitis-shows-robust-suppression-of-innate-and-adaptive-immune-pathways-with-tissue-repair-modulation/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeted-serum-proteomic-analysis-following-upadacitinib-treatment-in-ankylosing-spondylitis-shows-robust-suppression-of-innate-and-adaptive-immune-pathways-with-tissue-repair-modulation/