Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Tumor necrosis factor inhibitors (TNFi) are effective in treating patients with axial spondyloarthritis (axSpA), but they are associated with adverse effects and high costs. According to the ASAS-EULAR recommendations, if patients are in sustained remission or low disease activity, tapering of TNFi can considered.
We aimed to assess the risk of relapse after TNFi tapering strategies compared to standard dose continuation in patients with axSpA.
Methods:
We conducted a systematic search of the literature using Medline, Embase and Cochrane databases up to 27 February 2018. All randomized controlled trials (RCTs) and controlled cohort studies (CCTs) comparing the rate of relapse in patients with tapering dose versus standard dose of TNFi after achieving remission or low disease activity were selected. For the meta-analysis, the estimated event was the number of patients who had relapsed or not maintained remission or low disease activity in each treatment group (tapering versus standard dose). Data were extracted independently by two investigators. A global risk ratio (RR) was estimated using an inverse variance approach with fixed or random effect model, according to the level of heterogeneity (I2, Cochran’s Q-test). All these computations were performed using RevMan 5.3 software with a p-value threshold of 0.05.
Results:
Among the 544 publications screened, 5 studies (3 RCTs including one available only as abstract and 2 CCTs) were included, involving 230 patients who tapered TNFi dose and 226 treated with standard dose. Clinical heterogeneity between the trials was low: mean age between 46.0 and 46.7 years, male: 72.6% – 87.2%, ankylosing spondylitis according to modified New York criteria: 74% – 100%, HLA-B27 positive: 91.0% – 93.0%. Methodological heterogeneity between the trials was high: all tapering modalities, relapse definitions, duration of the follow-up and evaluation times were different. None of tapering strategies were disease activity guided.
Tapering TNFi dose was not associated with a statistically significant increase of relapse (RR [95% CI] = 1.51 [0.99 to 2.31], p = 0.05 in comparison with standard dose continuation [Figure]. A relapse was observed in 22.2% of patients who tapered TNFi versus 13.3% in patients with standard doses.
Conclusion:
Tapering doses of TNFi does not seem to increase the risk of relapse compared to TNFi standard dose continuation. However, data are scarce and heterogeneous, a need exists for additional, well designed, randomized controlled trials on this topic.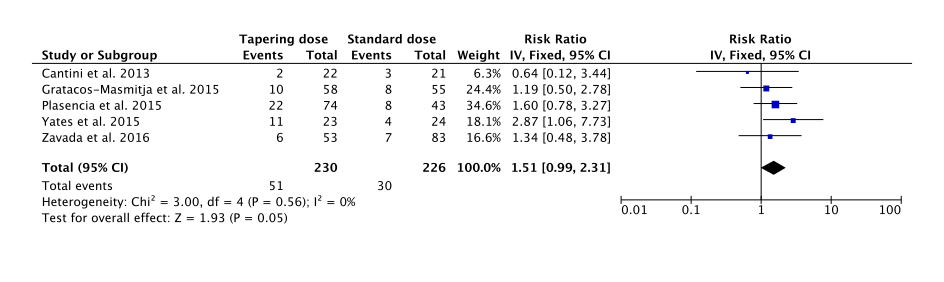
To cite this abstract in AMA style:
Couvaras L, Barnetche T, Constantin A, Pham T. Tapering TNF Inhibitors in Axial Spondyloarthritis: Systematicanalysisoftheliteratureandmeta-Analysis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/tapering-tnf-inhibitors-in-axial-spondyloarthritis-systematicanalysisoftheliteratureandmeta-analysis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tapering-tnf-inhibitors-in-axial-spondyloarthritis-systematicanalysisoftheliteratureandmeta-analysis/
