Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Gastrointestinal tract symptoms are common in systemic sclerosis (SSc). The Scleroderma Clinical Trials Consortium University of California Los Angeles Gastrointestinal Tract Questionnaire (GIT 2.0) is a validated, patient-reported outcome measure to assess gastrointestinal tract symptom severity in SSc. Intravital microscopy of the sublingual microcirculation can be used in SSc patient assessment for measurement of microvascular function and the glycocalyx, which is an indicator of endothelial dysfunction. In this study, biomarkers of endothelial dysfunction were examined in SSc patients by categorical GIT symptom severity.
Methods: SSc patients were enrolled at Vanderbilt University Medical Center and Tennessee Valley Healthcare System (IRB # 1618579).Enrolled patients who completed the GIT 2.0 and had same day sublingual microscopy measurement of glycocalyx penetrability by perfused boundary region [PBR]) in microvessel segments, and serum samples available for analysis were included. The PBR is scored as healthy (0-1.5), abnormal (1.6-3.5), or significantly elevated (3.6 and greater; indicative of glycocalyx dysfunction). The plasma measurements of serum glycocalyx turnover (hyaluronic acid [HA] and syndecan-1) were determined by ELISA and compared to age- and sex- matched healthy controls. The endothelial biomarkers (PBR, HA, and syndecan-1) were analyzed by total GIT 2.0 severity: none-to-mild (0.49) and moderate-to-severe (0.5-3.00).
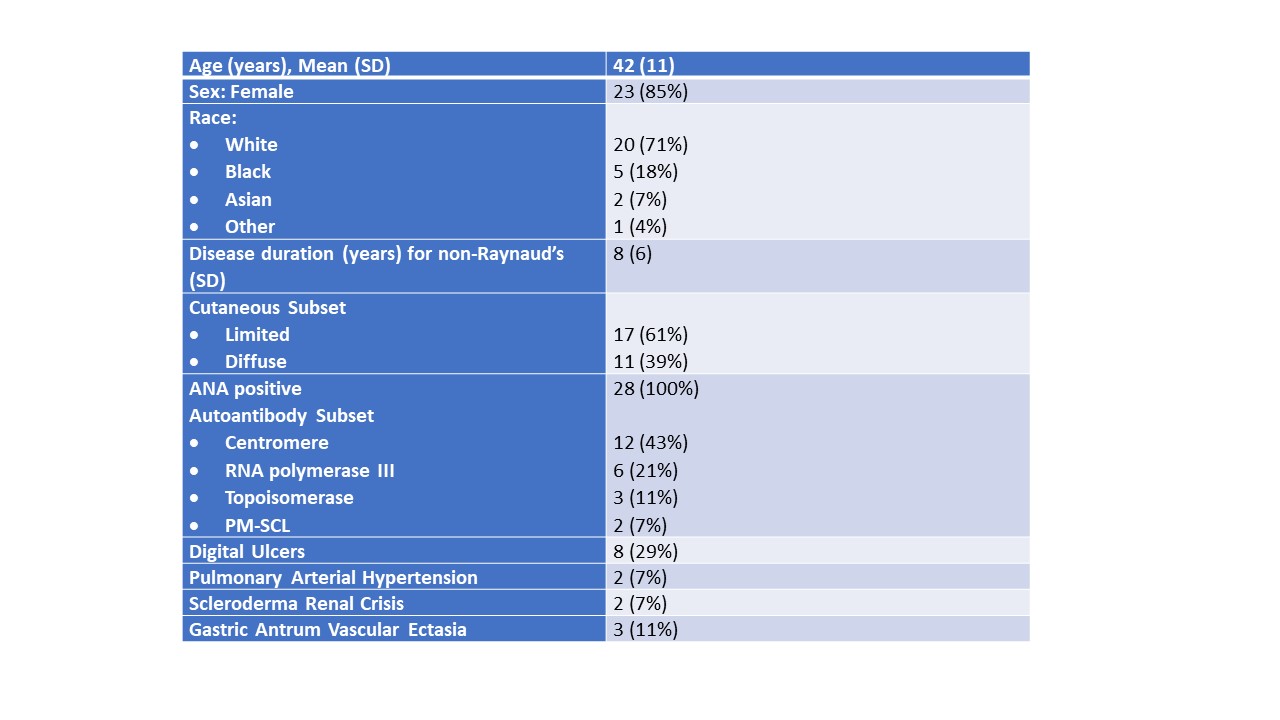
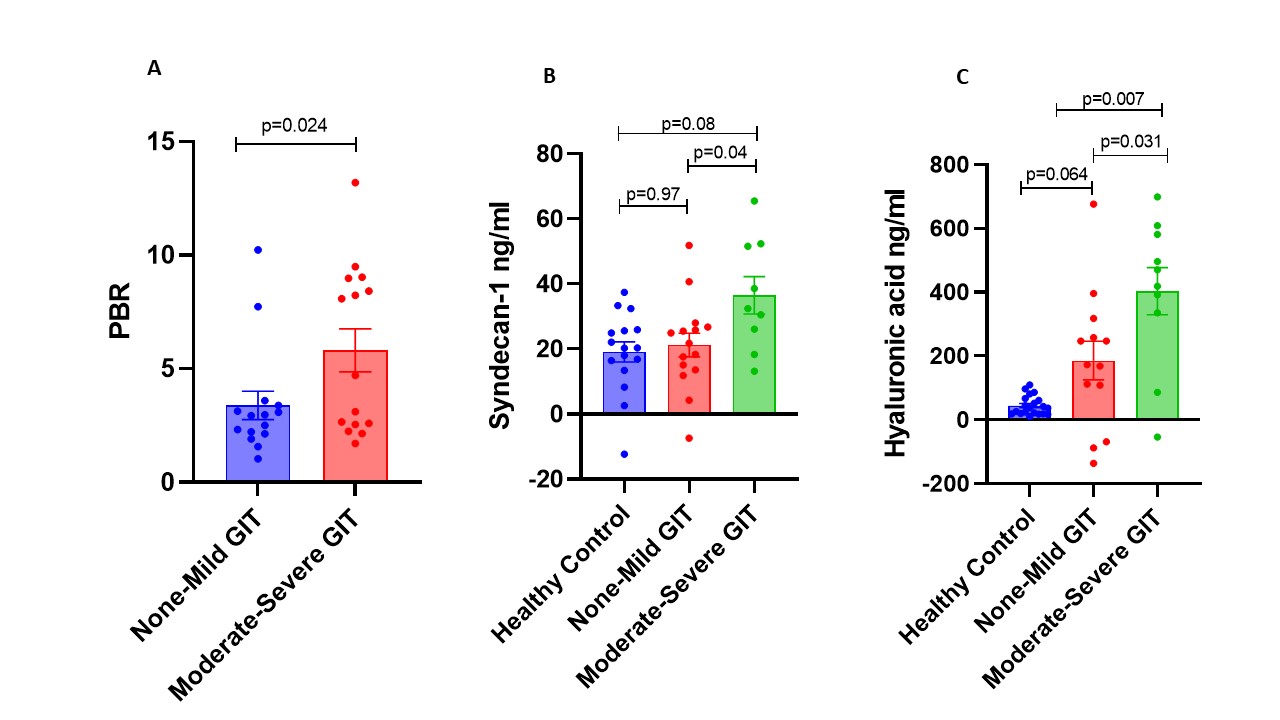
Results: The clinical features of 28 SSc patients that had same day acquisition of complete datasets are shown in Table 1. There were 15 SSc patients with mild GIT 2.0 and 13 SSc patients with moderate-to-severe GIT 2.0 symptoms, which were compared to 24 healthy control samples. The endothelial function biomarkers by GIT score category is shown in Figure 1. Only one patient had a healthy PBR measurement, 14 had an abnormal level, and 11 had an elevated PBR, which was significantly higher in patients with moderate-severe SSc-GIT symptoms (p=0.024). The syndecan -1 levels were significantly higher in SSc patients with moderate-severe GIT symptoms compared to patients with mild GIT symptoms (p=0.04), but not healthy controls. The HA levels were significantly higher in patients with moderate-severe SSc-GIT symptoms compared to both SSc mild GIT symptoms (p=0.031) and healthy controls (p=0.007).
Conclusion: This study supports that GIT symptoms in SSc may be due to endothelial dysfunction. The glycocalyx is a multifunctional and dynamic structure that participates in many vascular processes, including but not limited to vascular permeability, inflammation, thrombosis, mechano-transduction, and cytokine signaling. Sublingual estimates of endothelial glycocalyx health and serum measures of HA and syndecan-1 support the potential use of these biomarkers for objectively quantifying the role of vasculopathy in symptomatic SSc-GIT disease.
To cite this abstract in AMA style:
Gogulamudi V, Wood S, Johnson E, Petrey A, Donato A, Frech T. Systemic Sclerosis Gastrointestinal Tract Vascular Biomarkers of Symptomatic Disease Activity [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/systemic-sclerosis-gastrointestinal-tract-vascular-biomarkers-of-symptomatic-disease-activity/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/systemic-sclerosis-gastrointestinal-tract-vascular-biomarkers-of-symptomatic-disease-activity/