Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Elevated levels of RA-associated autoantibodies (ACPA, RF) prior to the clinical onset of inflammatory arthritis (IA) define a state of risk for future development of RA. Despite elevated autoantibodies indicating a loss of immune tolerance, we possess a limited understanding of the immune events regulating this state, including the molecular processes that contribute to the progression to clinical RA. This mechanistic gap impedes the design of effective preventative interventions.
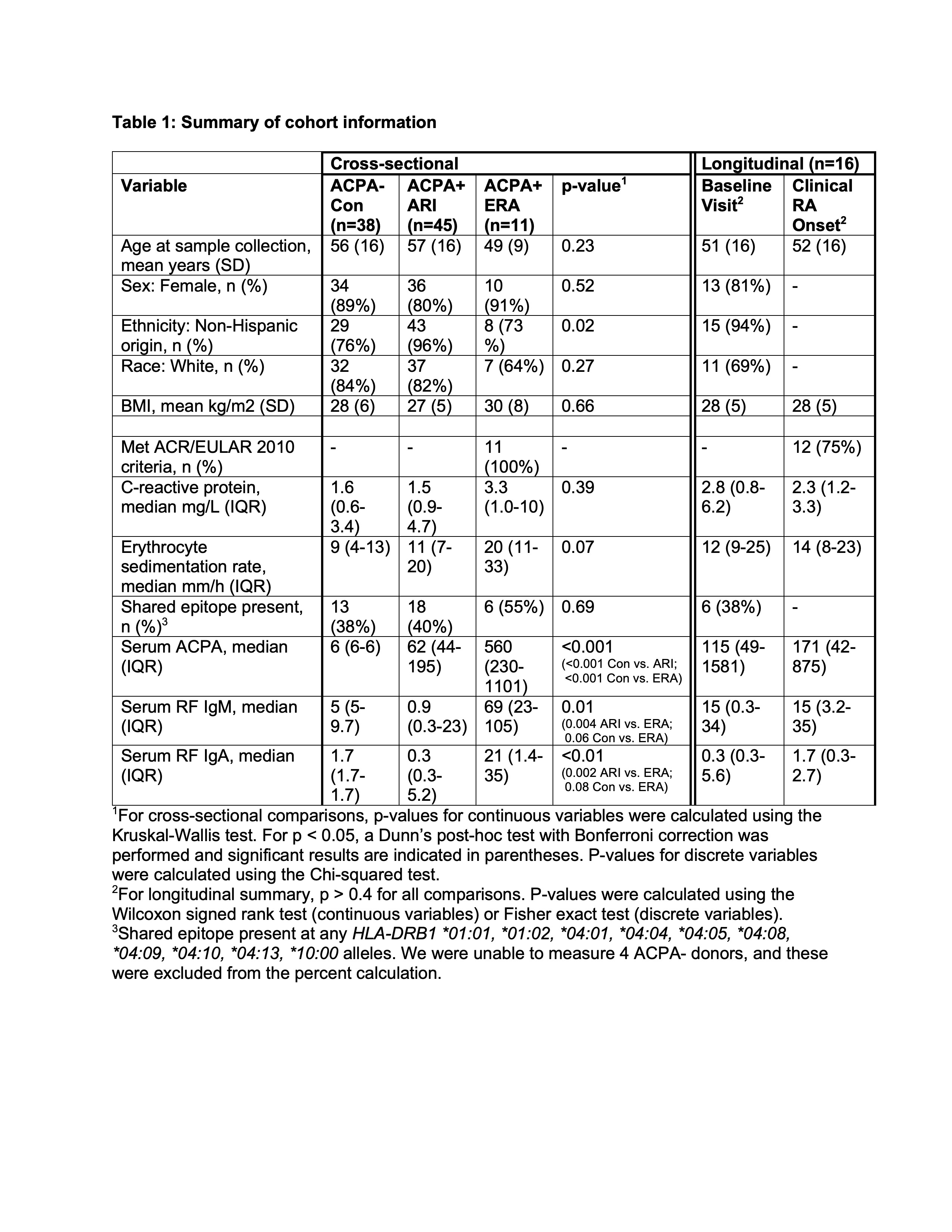
Methods: To address this gap, we performed a multi-omics longitudinal analysis of the plasma proteome, peripheral blood mononuclear cell (PBMC) transcriptomes by scRNA-seq and surface phenotypes by flow cytometry. Samples were from a prospective longitudinal cohort study of ACPA+ at-risk individuals (ARI) without baseline IA (Table 1). We performed longitudinal modeling in participants who progressed to clinical RA (n=16). We supplemented longitudinal analyses with cross-sectional analyses of baseline ACPA+ ARI (n=45), ACPA- controls (Con; n=38) and ACPA+ early RA (ERA) < 1yr from clinical diagnosis (n=11).
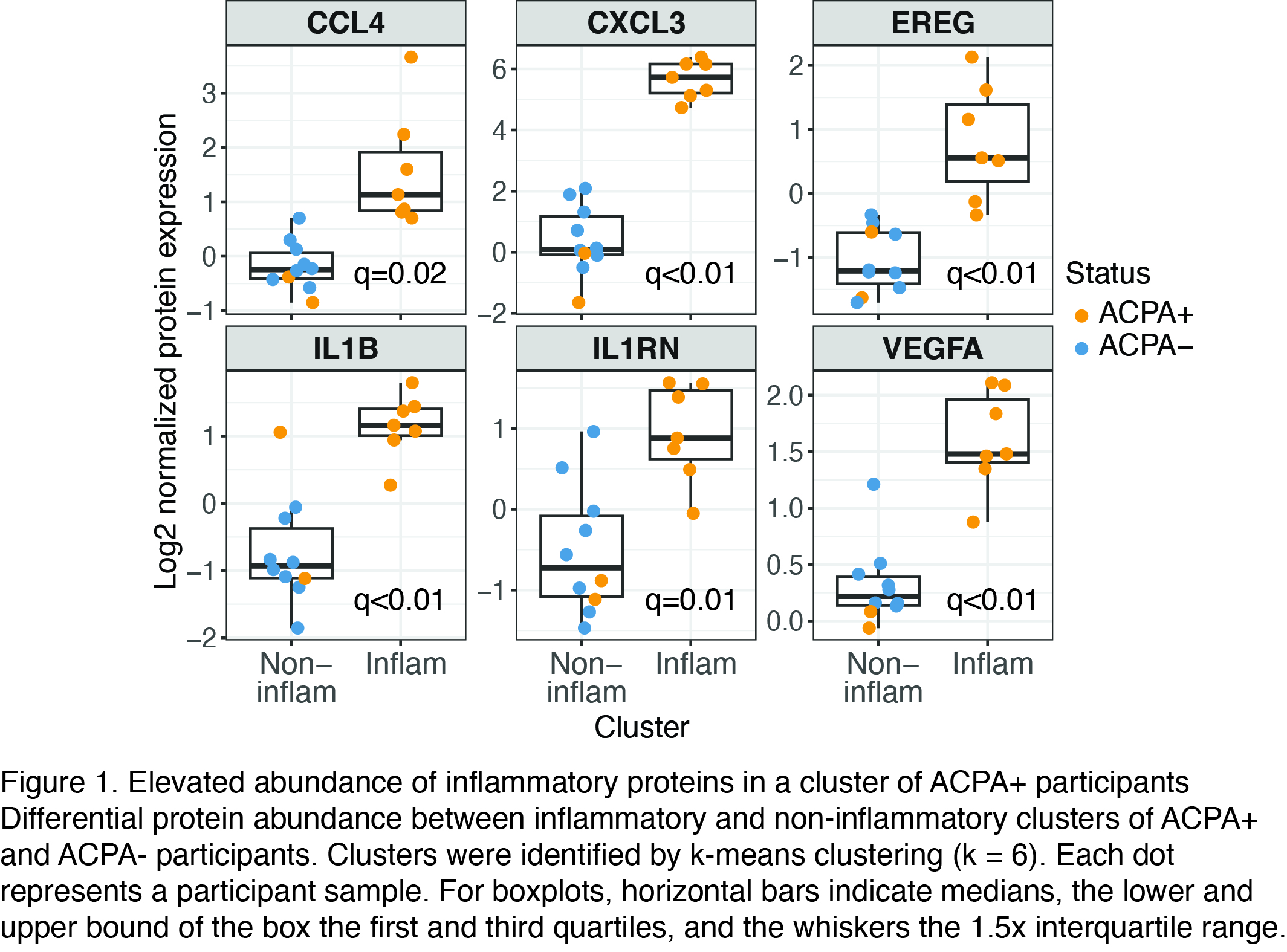
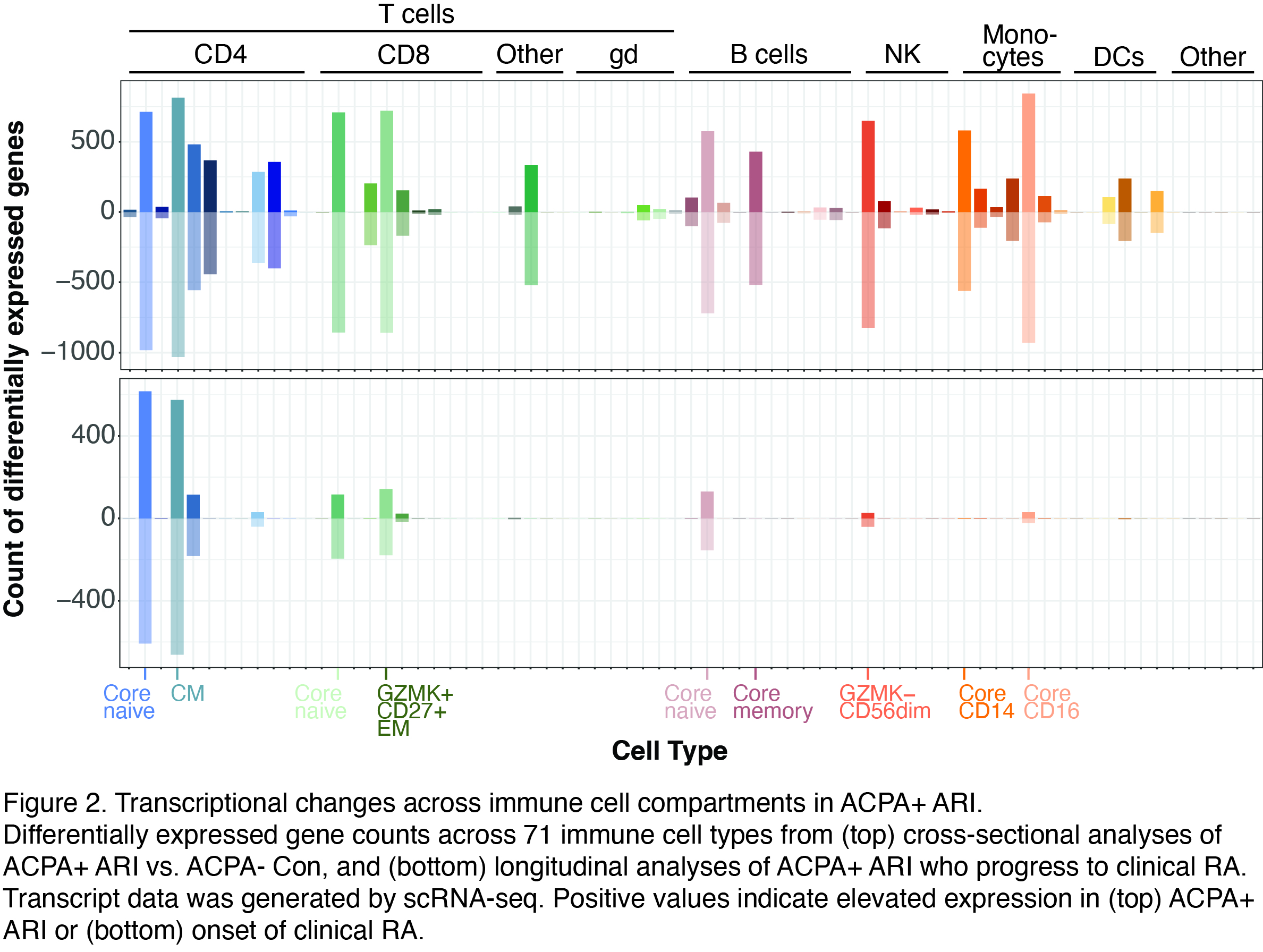
Results: We identified 275 proteins that differentiated ACPA+ ARI from ACPA- Con. By k-means clustering, we identified an inflammatory protein signature that included elevated IL1B, VEGFA, IL1RN, CXCL3, EREG and CCL4 in a cluster of ACPA+ donors (Fig. 1), which overlaps a signature from an inflammatory subset of tissue monocytes identified in prior work (Mulder Immunity 2021). The inflammatory protein signature was accompanied by transcriptional reprogramming across all major immune compartments, and in both naïve and effector memory lymphocyte populations of ACPA+ ARI (Fig. 2). By contrast, in longitudinal samples of ACPA+ ARI who developed clinical RA, we observed a diminished transcriptional response. Only CD4 naïve and central memory (CM) T cells showed a similar number of transcriptional changes to the cross-sectional ACPA+ ARI vs. ACPA- Con comparison (Fig. 2). We discovered a shared activation signature within CD4 naïve and CM T cells during progression to clinical RA, highlighted by reduced expression of CD3 components of the T cell receptor complex (CD3G, q=0.01) together with elevated STAT5B (q=0.03), ICOS (q=0.04) and AKT3 (q=0.04). These progressive changes in naive and CM T cells were the same that were diminished in our independent analyses of data from RA patients who responded to CTLA4-Ig therapy from Iwasaki et al. (Arthritis Res Ther 2024), supporting potential clinical significance.
Conclusion: ACPA+ ARI exhibit molecular signatures of inflammation and widespread transcriptional reprogramming within the circulation despite the absence of arthritis. Naïve and CM CD4 T cells display a shared activation program during progression to clinical RA. The penetrance of this shared activation signature suggests it could be a result of the inflammatory milieu, which is consistent with our proteomics observations. Our findings provide key insights into mechanisms contributing to immune dysregulation in the ACPA+ state and progression to clinical RA, and may suggest targets for early preventative intervention or molecular endpoints for RA prevention trials.
To cite this abstract in AMA style:
Gillespie M, He Z, Savage A, Venkatesan P, Glass M, Okada L, Tran N, He Y, Rachid Zaim S, Ravisankar P, Bennett C, Reading J, Garber J, Genge P, Hernandez V, Heubeck A, Kawelo E, Krishnan U, Lee K, Mettey R, Musgrove B, Parthasarathy V, Phalen C, Roll C, Stuckey T, Weiss M, Gustafson C, Gong Q, Kuan E, Peng T, Graybuck L, Demoruelle K, Kuhn K, Boyle D, Zhang F, Bumol T, Goldrath A, Li X, Holers V, Skene P, Firestein G, Deane K, Torgerson T. Systemic Inflammation and Transcriptional Reprogramming Contribute to Progression to Active Rheumatoid Arthritis in ACPA+ Individuals [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/systemic-inflammation-and-transcriptional-reprogramming-contribute-to-progression-to-active-rheumatoid-arthritis-in-acpa-individuals/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/systemic-inflammation-and-transcriptional-reprogramming-contribute-to-progression-to-active-rheumatoid-arthritis-in-acpa-individuals/