Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis (RA) is an incurable autoimmune disease where response to therapy is heterogenous. Clinically indistinguishable RA patients can be classified histo-pathologically into three distinct pathotypes: lympho-myeloid (LM), diffuse-myeloid (DM) and fibroid-pauci-immune (FPI). They are characterized by heavy lymphocytic infiltration (LM), macrophage rich / lymphoid poor (DM) and fibroblast rich / myeloid and lymphoid poor (FPI). Understanding the signaling pathways associated with these pathotypes is likely to be critical for therapeutic targeting. Our pilot (phospho)proteome analysis of 8 early-stage RA synovial biopsies highlighted that immunological pathways were preferentially associated with LM compared to DM with distinct phosphorylated kinases1. Here, to establish the dynamics of expression across disease stages, we expanded that analysis to advanced, anti-TNF inadequate responder RA patients recruited to the R4RA trial2,3.
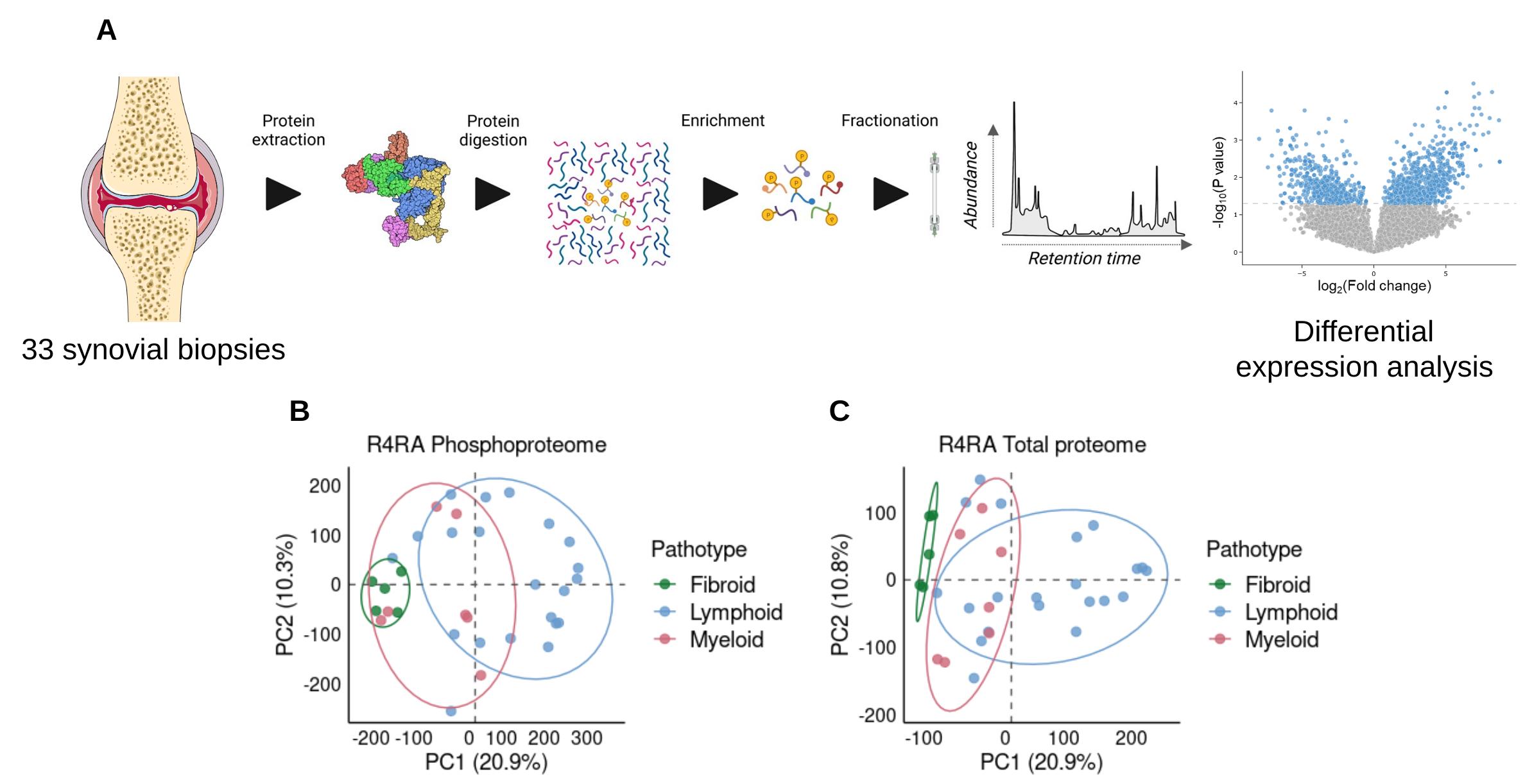
Methods: The phosphoproteome and proteome profiles of 33 pre-treatment synovial biopsies from the R4RA trial were analyzed using label-free mass spectrometry (Figure 1A). There were 21 lympho-myeloid, 7 diffuse-myeloid and 5 fibroid-pauci-immune samples.
Results: Principal component analysis highlighted that synovial pathotypes are key drivers of the phosphoproteome and proteome profiles (Figure 1B-C). Lympho-myeloid was the most immunologically active with the upregulation of lymphoproliferative signaling pathways, recapitulating the pilot study with lymphoid and myeloid samples1. This larger scale analysis demonstrated an enrichment of B cell receptor related kinases, such as B Lymphocyte Kinase (BLK, p = 0.001) in LM compared to DM pathotype. Furthermore, the gradient of immune signaling across the three pathotypes (LM > DM > FPI) is also seen through the expression of Mammalian Target of Rapamycin (MTOR) where MTOR and 3-Phosphoinositide Dependent Protein Kinase 1 (PDPK1) were upregulated in LM compared to FPI (p = 0.001 and 0.009, respectively), whereas PDPK1 was enriched in DM compared to FPI (p = 0.008). Conversely, the FPI (phospho)proteome signatures were associated with cytoskeletal and extracellular matrix processes (e.g., vimentin and collagen proteins), consistent with known fibroblast function.
Conclusion: Synovium (phospho)proteome analysis identified specific signaling pathways associated with distinct pathotypes both in early and advanced RA, which can be exploited for targeted therapeutic applications. R4RA treatment response analysis is ongoing.
References
1Çubuk, C. et al. (2024), Arthritis Research & Therapy, 26(1). doi:10.1186/s13075-024-03351-4.
2Humby, F. et al. (2021), The Lancet, 397(10271), pp. 305–317. doi:10.1016/s0140-6736(20)32341-2.
3Rivellese, F. et al. (2022), Nature Medicine, 28(6), pp. 1256–1268. doi:10.1038/s41591-022-01789-0.
Acknowledgements
https://www.nature.com/articles/s41591-022-01789-0#Ack1
(A) 33 pre-treatment synovial biopsies from the R4RA trial underwent protein extraction, digestion and fractionation for mass spectrometry analysis of the phosphoproteome and proteome profile. With phosphoproteome analysis, there was an additional enrichment step to isolate the phosphoproteins. The workflow diagram is adapted from Higgins et al Biochemical Journal 2023. The joint image is from ServierMedical Art, licensed under a Creative Commons Attribution 3.0 unported license. (B)Principal component analysis (PCA) of the phosphoproteome profiles and (C)total proteome profiles.
To cite this abstract in AMA style:
Cubuk C, Lau R, Rajeeve V, Cutillas P, Hands R, Fossati-Jimack L, Surace A, Nerviani A, Rivellese F, Lewis M, Pitzalis C. Synovial Phosphoproteomic Profiling in Early and Advanced Rheumatoid Arthritis (R4RA Trial) Identifies Specific Signaling Pathways Linked to Distinct Pathotypes [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/synovial-phosphoproteomic-profiling-in-early-and-advanced-rheumatoid-arthritis-r4ra-trial-identifies-specific-signaling-pathways-linked-to-distinct-pathotypes/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/synovial-phosphoproteomic-profiling-in-early-and-advanced-rheumatoid-arthritis-r4ra-trial-identifies-specific-signaling-pathways-linked-to-distinct-pathotypes/