Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Ankylosing spondylitis (AS) is a chronic inflammatory disease of the axial skeleton associated with pain, stiffness, and disability.1 Up to 66% of patients (pts) with AS may also have peripheral involvement, including swollen and tender joints (STJs),2,3 which are associated with worse overall disease activity.4 A previous analysis showed that secukinumab, a selective inhibitor of interleukin 17A, led to significant improvements in efficacy outcomes vs placebo, regardless of peripheral joint involvement.3 However, the effect of secukinumab on symptoms of peripheral arthritis in pts with AS was not assessed.
The objective of this analysis was to assess changes in peripheral symptoms in pts with AS treated with secukinumab vs placebo.
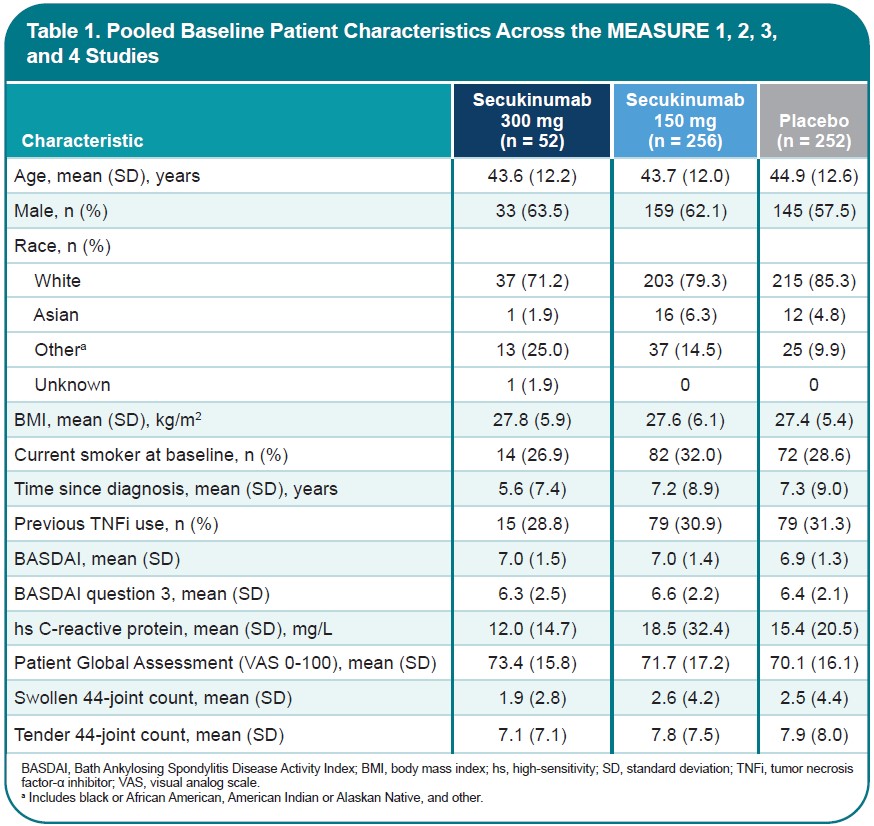
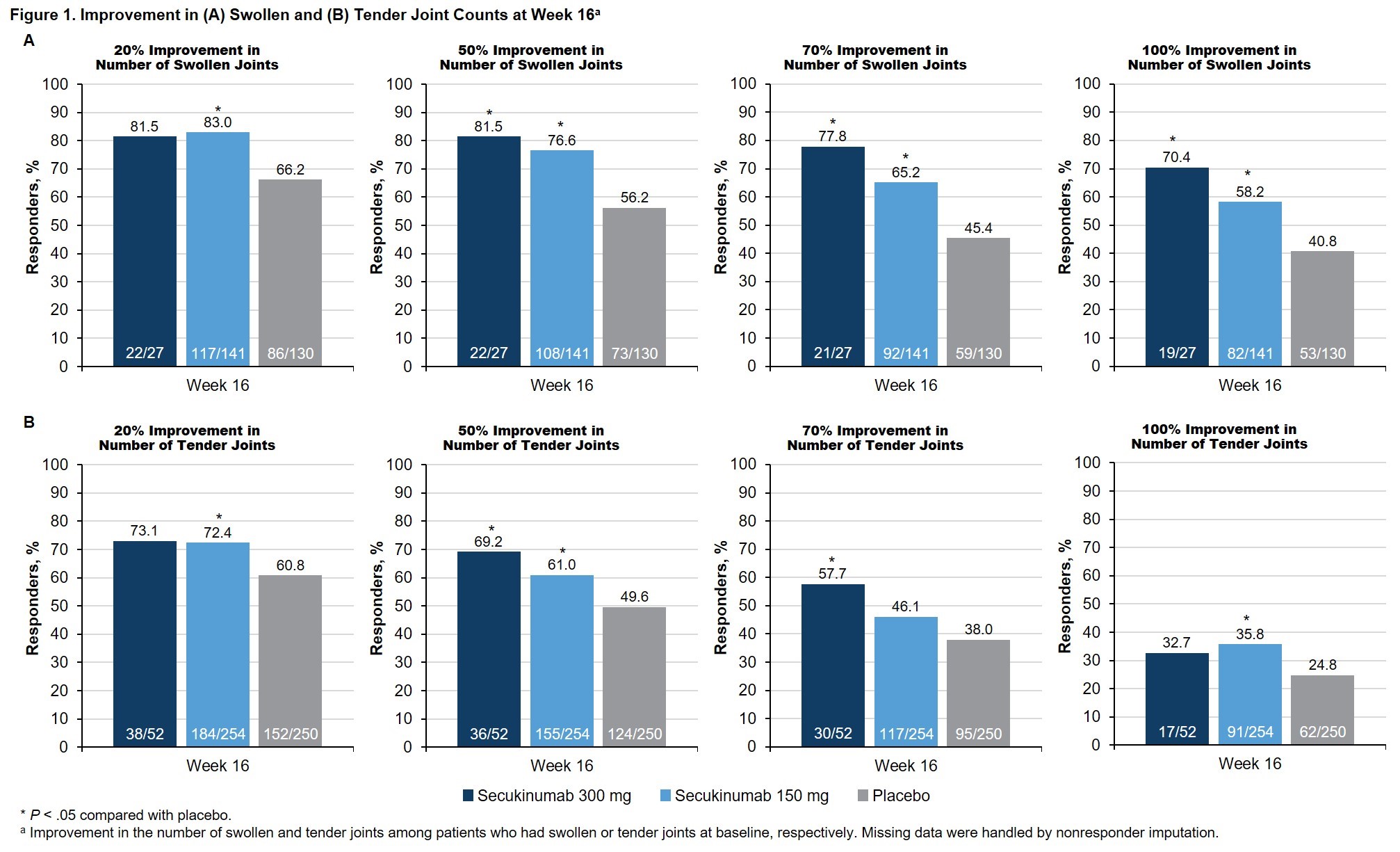
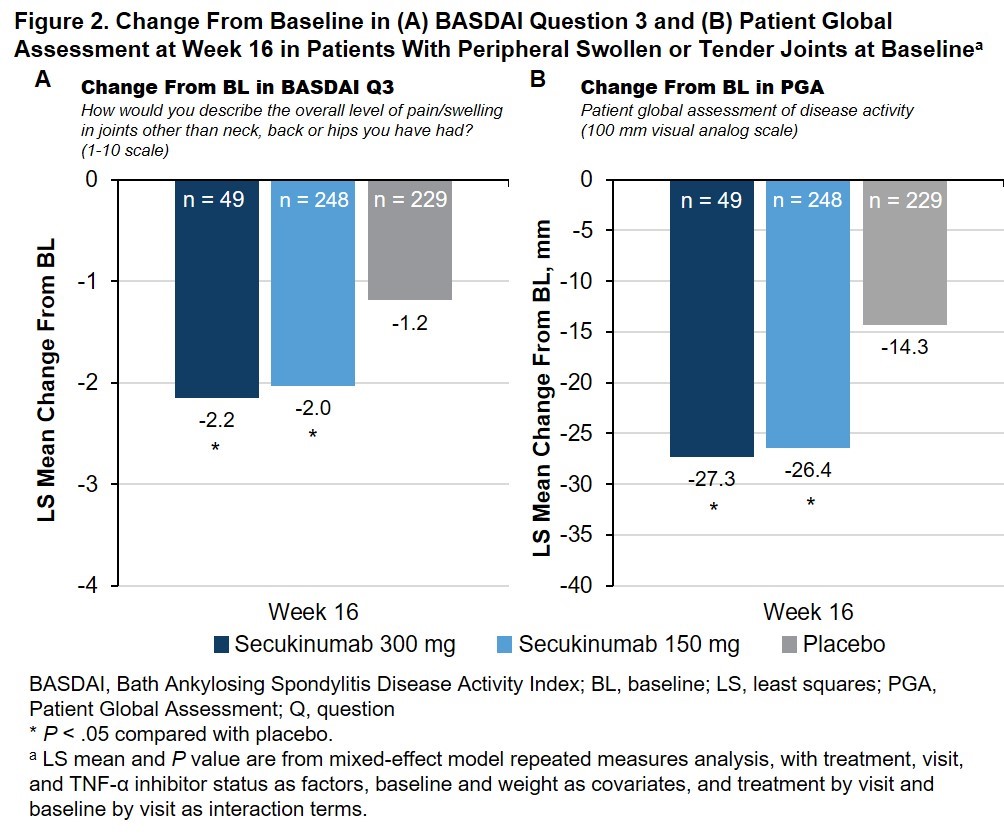
Methods: Data from pts with active AS and peripheral symptoms who were enrolled in MEASURE 1 (NCT01358175), 2 (NCT01649375), 3 (NCT02008916), and 4 (NCT02159053) were pooled in this hypothesis-generating analysis. No adjustments for multiple comparisons were made. Pts with peripheral symptoms were identified by the presence of STJs, based on 44-joint counts at baseline (BL). Pts received subcutaneous (SC) secukinumab every 4 weeks at doses of 300 mg with an intravenous (IV) loading dose (MEASURE 3 only), 150 mg with an IV or SC loading dose, or placebo. Treatment response through Week 16 was assessed based on the proportions of pts who achieved improvements of 20%, 50%, 70%, or 100% in the number of swollen and number of tender joints and improvements in the BASDAI score for question 3 and Patient Global Assessment (PGA). Changes in the number of swollen and number of tender joints were assessed in pts with swollen or tender joints at BL, respectively.
Results: This pooled analysis included 560 pts with AS and STJs at BL (Table). At Week 16, treatment with secukinumab led to significantly greater proportions of pts achieving reductions in the number of swollen (Fig 1A) or tender (Fig 1B) joints compared with placebo; the treatment effect was more pronounced in reduction of swollen joints. Furthermore, a greater proportion of secukinumab-treated pts achieved complete resolution of swollen or tender joints vs placebo (Fig 1). Secukinumab also led to significant improvements in peripheral pain/swelling (Fig 2A) and disease activity (Fig 2B) vs placebo, as assessed using BASDAI question 3 and the PGA, respectively.
Conclusion: In parallel with its previously reported efficacy in axial symptoms,3 secukinumab led to significant improvements in symptoms of peripheral arthritis in pts with AS. Significant improvements were seen in both tender and swollen joints.
References:
- Braun J, Sieper J. Lancet. 2007;369:1379-1390.
- de Winter JJ, et al. Arthritis Res Ther. 2016;18:196.
- Mease P, et al. Arthritis Rheumatol. 2019;71(suppl 10):1553.
- de Winter JJ, et al. RMD Open. 2019;5:e000802.
To cite this abstract in AMA style:
Mease P, Deodhar A, Calheiros R, Meng X, Fox T, Baraliakos X. Symptoms of Peripheral Arthritis Are Significantly Improved in Patients with Ankylosing Spondylitis Treated with Secukinumab [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/symptoms-of-peripheral-arthritis-are-significantly-improved-in-patients-with-ankylosing-spondylitis-treated-with-secukinumab/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/symptoms-of-peripheral-arthritis-are-significantly-improved-in-patients-with-ankylosing-spondylitis-treated-with-secukinumab/