Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: In the phase 3 BE MOBILE 1 and 2 studies, bimekizumab (BKZ), a monoclonal IgG1 antibody that inhibits interleukin (IL)-17F in addition to IL-17A, demonstrated sustained improvements in physical function and health-related quality of life (HRQoL) to Week (Wk) 52 in patients (pts) with non-radiographic (nr-) and radiographic axial spondyloarthritis (r-axSpA; i.e., AS).1 We report data to 2 years for these outcomes, in addition to spinal mobility, from the ongoing open‑label extension (OLE) study, BE MOVING.
Methods: BE MOBILE 1 (NCT03928704) and 2 (NCT03928743) both comprised a 16-wk double-blind period followed by a 36-wk maintenance period. Pts were randomized to subcutaneous BKZ 160 mg every 4 wks (Q4W) or placebo (PBO); from Wk 16, all received BKZ 160 mg Q4W. At Wk 52, eligible participants could enrol in the combined OLE study, BE MOVING (NCT04436640).
We report scores for Bath AS Metrology Index (BASMI; 0–10 numerical rating scale assessing spinal mobility), Bath AS Functional Index (BASFI; 0–10 numerical rating scale assessing physical function), Short Form-36 Physical Component Summary (SF-36 PCS; mean/standard deviation in United States [US] general population: 50/10),2 individual SF-36 domains, and AS Quality of Life (ASQoL; score range: 0–18) to Wk 104 using multiple imputation (MI). We also report the proportion of pts achieving BASFI ≤2 and clinically meaningful ASQoL improvement (reduction of ≥4 points in pts with baseline score ≥4) to Wk 104 (non-responder imputation [NRI] and observed case [OC]). Data are pooled for all randomized pts with nr-axSpA and r-axSpA in BE MOBILE 1 and 2.
Results: Of the pts originally randomized in BE MOBILE 1 (nr-axSpA) and BE MOBILE 2 (r-axSpA), 81.9% (208/254) and 86.1% (286/332) pts entered BE MOVING at Week 52, respectively; 189 nr-axSpA pts and 267 r-axSpA pts completed Wk 104.
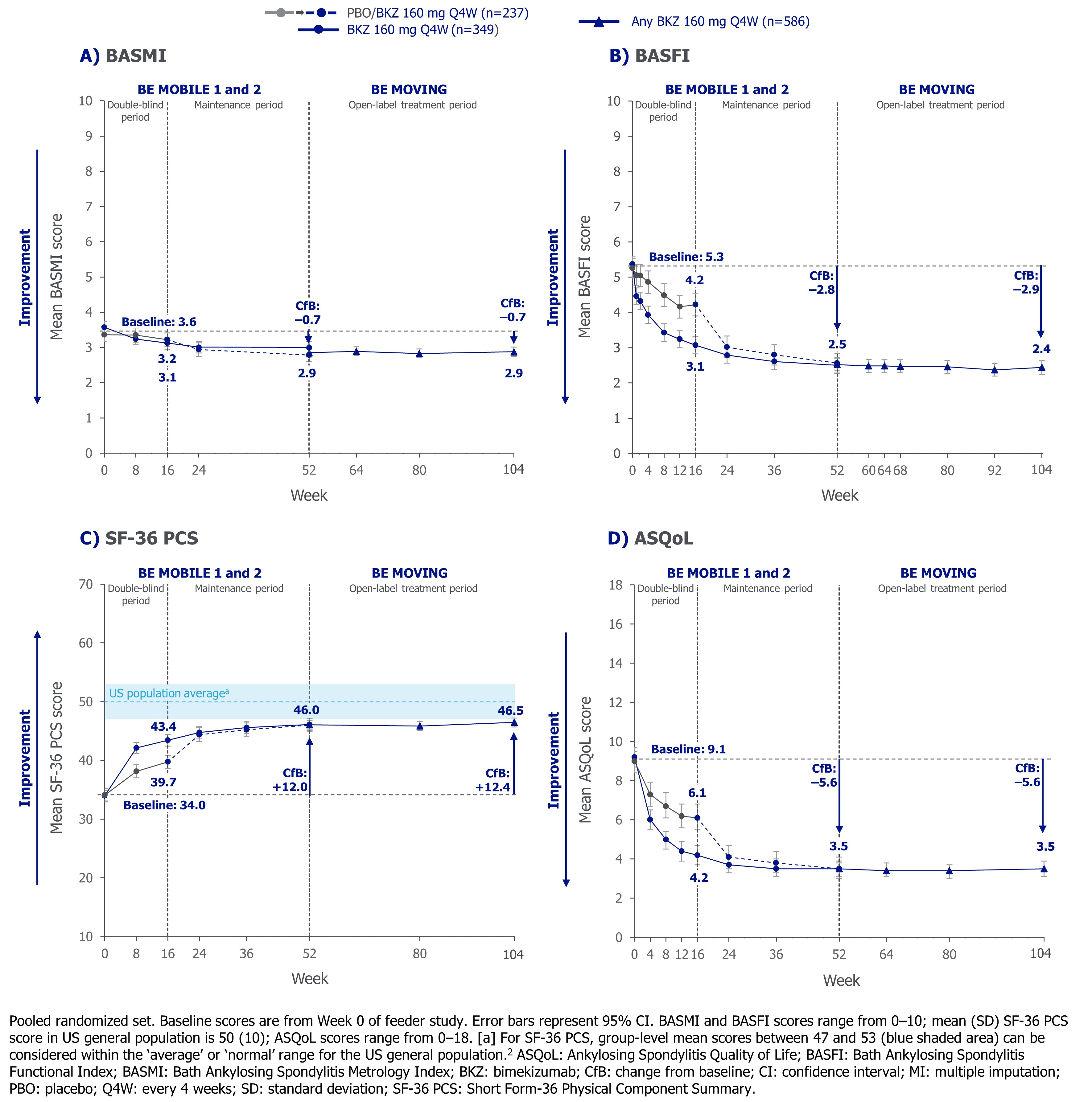
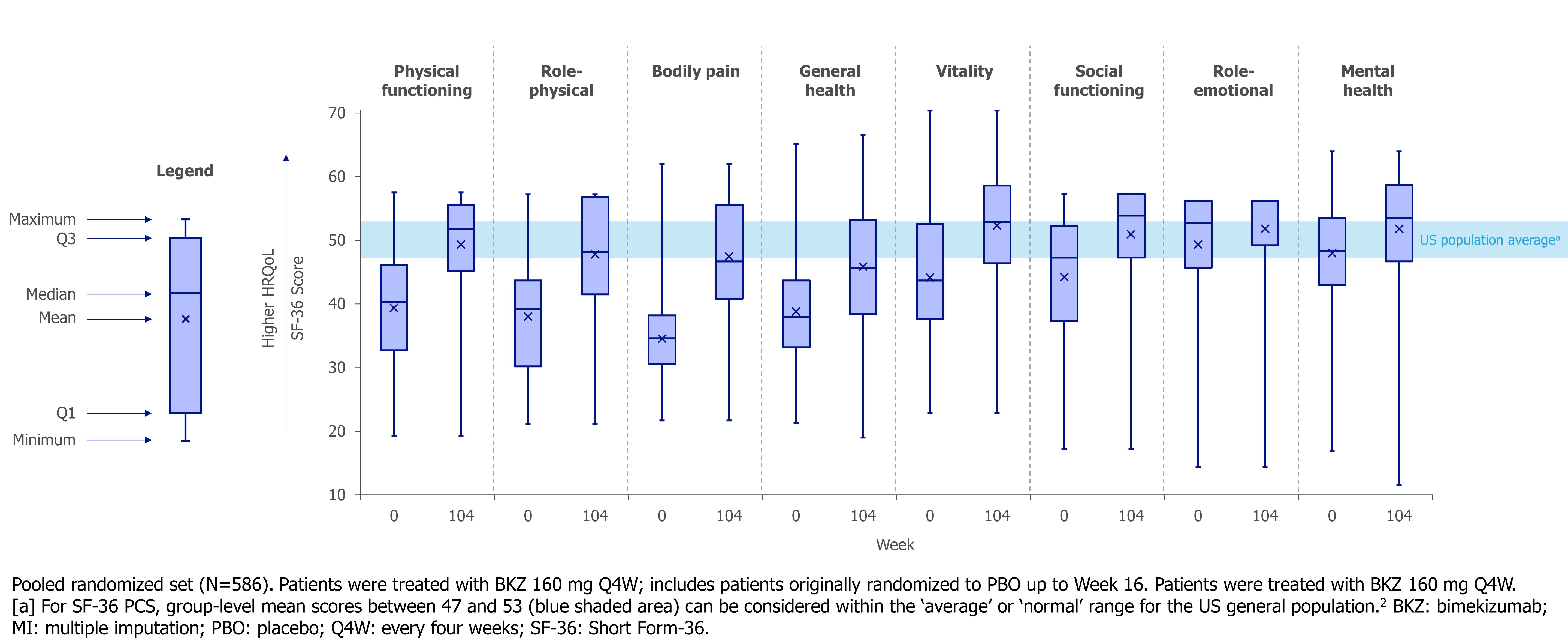
Mean reductions from baseline with BKZ seen at Wk 52 in BASMI, BASFI, and ASQoL were sustained to Wk 104 (Wk 52: –0.6, –2.8, and –5.6; Wk 104: –0.6, –2.9, and –5.6, respectively; Figure 1). Mean improvement from baseline seen at Wk 52 in SF-36 PCS was also sustained to Wk 104 (Wk 52: +12.0; Wk 104: +12.4; Figure 1). After 2 years of BKZ treatment, the mean SF-36 PCS score approached the lower bound of the US population average (Figure 1).2 Improvements across all individual SF-36 domains were observed from baseline to Wk 104 (Figure 2).
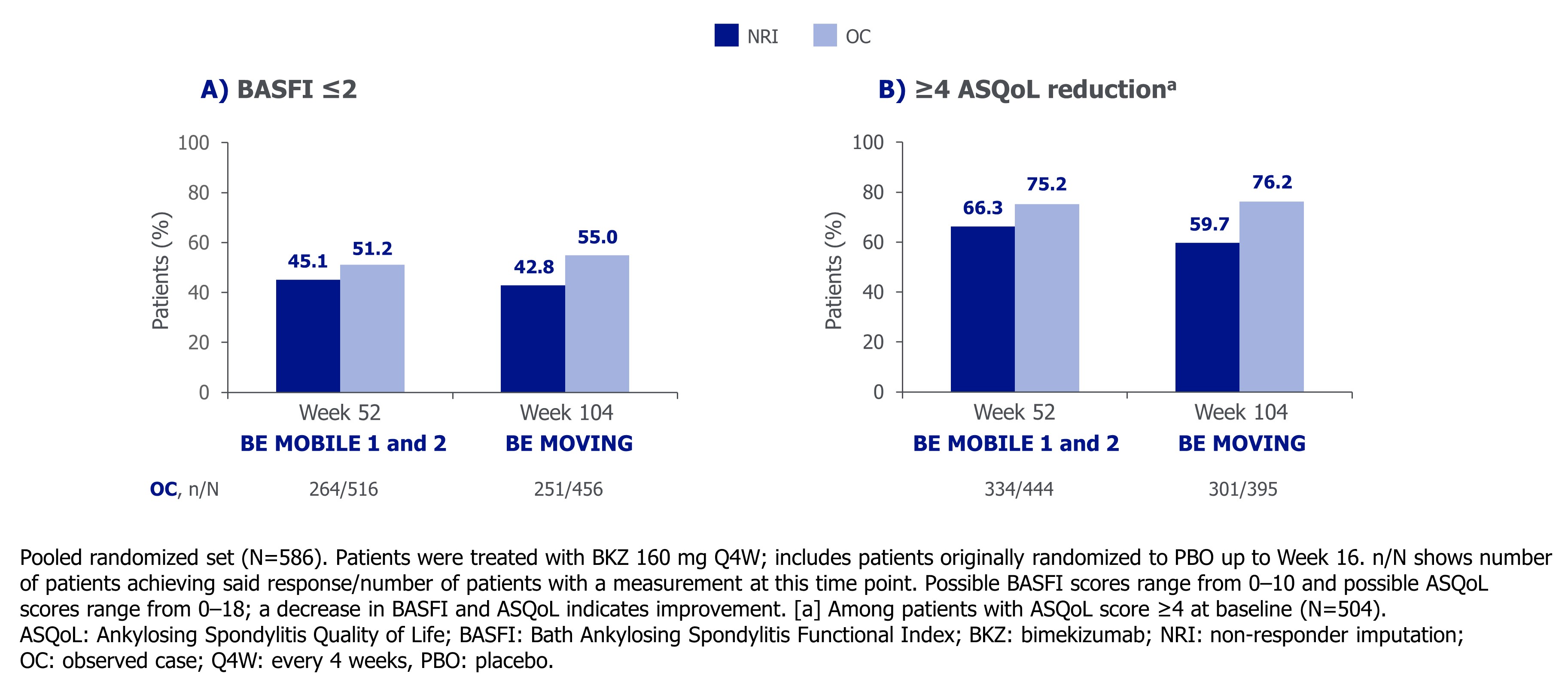
The proportions of pts that achieved BASFI ≤2 or clinically meaningful ASQoL improvement at Wk 52 were sustained to Wk 104 in BE MOVING (BASFI ≤2 Wk 52: 45.1%, Wk 104: 42.8%; ≥4 ASQoL reduction Week 52: 66.3%, Wk 104: 59.7% [NRI]; Figure 3). Results were similar across nr-axSpA and r-axSpA pts.
Conclusion: BKZ treatment resulted in sustained improvements in spinal mobility, physical function, and HRQoL over 2 years across the full disease spectrum of axSpA.
References: 1. Dubreuil M. ACR 2023. Poster 0526; 2. Maruish ME. User’s manual for the SF-36v2 Health Survey (3rd ed.).
To cite this abstract in AMA style:
Navarro Compán V, Dubreuil M, Gaffney K, Kay J, de la Loge C, Massow U, Taieb V, Vaux T, Deodhar A. Sustained Improvements with Bimekizumab in Spinal Mobility, Physical Function and Health-Related Quality of Life in Patients with Axial Spondyloarthritis: 2-Year Results from Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/sustained-improvements-with-bimekizumab-in-spinal-mobility-physical-function-and-health-related-quality-of-life-in-patients-with-axial-spondyloarthritis-2-year-results-from-two-phase-3-studies/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustained-improvements-with-bimekizumab-in-spinal-mobility-physical-function-and-health-related-quality-of-life-in-patients-with-axial-spondyloarthritis-2-year-results-from-two-phase-3-studies/